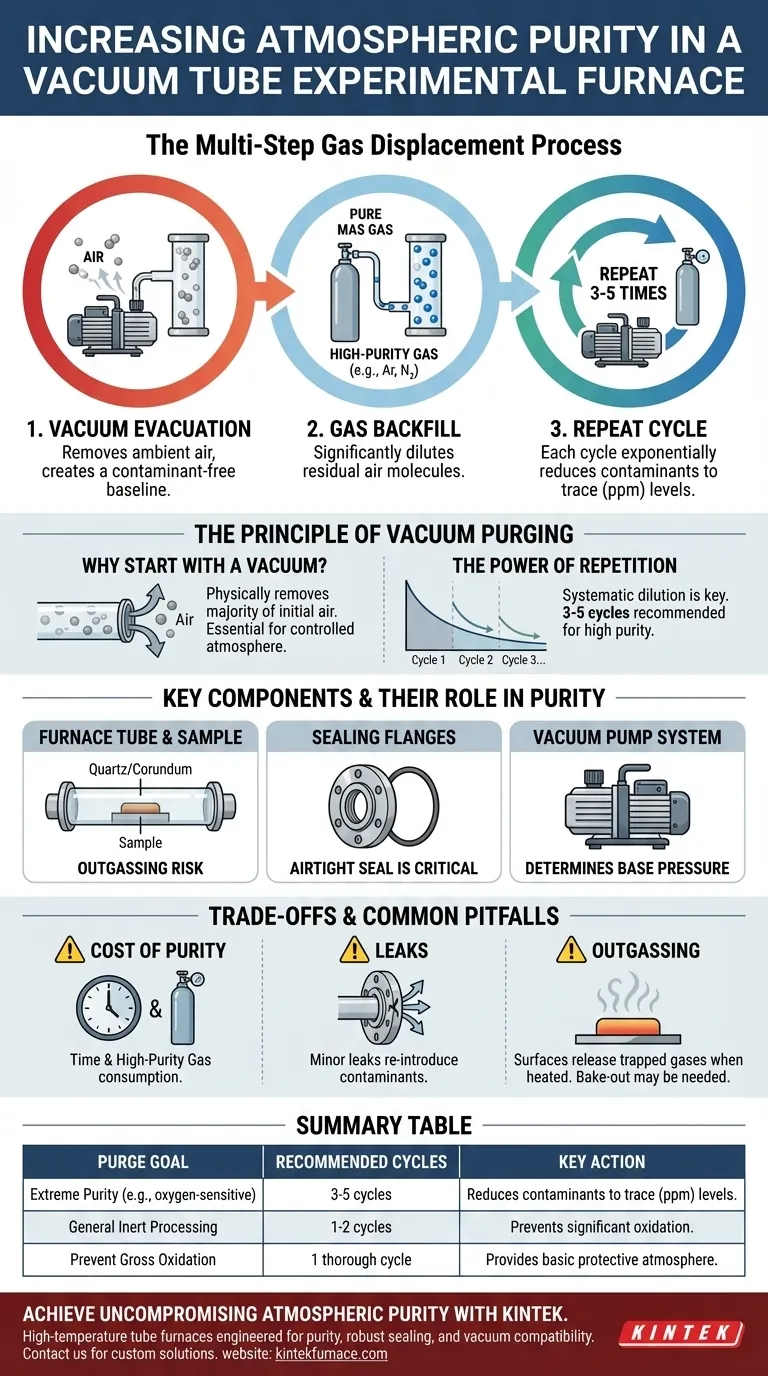
To achieve the highest atmospheric purity in a vacuum tube experimental furnace, you must perform a multi-step gas displacement process. First, use a vacuum pump to remove the existing ambient air from the sealed furnace tube. Then, introduce your desired high-purity atmosphere. For optimal results, this cycle of evacuating the tube and refilling it with the process gas must be repeated several times.
The core principle is not just about replacing the air once, but about systematically diluting the remaining contaminants. Each cycle of pumping a vacuum and backfilling with pure gas exponentially reduces the concentration of unwanted molecules like oxygen and moisture, leading to a progressively purer environment.

The Principle of Vacuum Purging
Achieving a high-purity atmosphere is a process of active contamination removal. The air initially present in the furnace tube is the primary contaminant that must be displaced before any thermal processing begins.
Why Start with a Vacuum?
The initial step of pumping the furnace tube into a vacuum physically removes the vast majority of the ambient air molecules. This creates a contaminant-free baseline that is essential for establishing a controlled atmosphere. Without this step, you would simply be mixing your process gas with the existing air.
The First Backfill: A Dilution Step
After the initial evacuation, the tube is backfilled with your high-purity process gas (e.g., argon, nitrogen). This step significantly dilutes any residual air molecules that the vacuum pump could not remove. However, a single cycle is often insufficient for sensitive experiments.
The Power of Repetition: The Purge Cycle
The most critical step for achieving high purity is repeating the process. Each subsequent cycle—evacuating the diluted gas mixture and refilling with fresh, pure gas—removes a large fraction of the remaining contaminants. Three to five purge cycles are often recommended to reduce contaminant levels to the parts-per-million (ppm) range.
Key Components and Their Role in Purity
The effectiveness of the vacuum purging process is entirely dependent on the integrity of the furnace system's components. A failure in any one part can compromise the entire procedure.
The Furnace Tube and Sample
The experimental material is placed inside a sealed quartz or corundum tube. The choice of tube material is critical for temperature and chemical compatibility, but its cleanliness also impacts purity. The tube and the sample itself can release adsorbed gases when heated, a phenomenon known as outgassing.
The Sealing Flanges
The furnace tube is typically sealed at both ends with stainless steel flanges. These flanges and their O-rings create the airtight seal necessary to hold a vacuum and prevent ambient air from leaking back into the system. A perfect seal is non-negotiable for maintaining purity.
The Vacuum Pump System
The quality of your vacuum pump determines the "base pressure"—the lowest pressure it can achieve. A more powerful pump (or a combination of pumps) removes more of the initial air, providing a cleaner starting point and making each subsequent purge cycle more effective.
Understanding the Trade-offs and Common Pitfalls
While the vacuum purge method is highly effective, it is essential to understand its practical limitations and potential sources of error.
The Cost of Purity: Time and Gas
Each purge cycle consumes both time and expensive, high-purity gas. You must balance the level of atmospheric purity required for your experiment against these practical costs. Not every process requires five purge cycles.
The Persistent Threat of Leaks
The entire procedure is rendered ineffective by even a minor leak in the system, most commonly at the flange seals. A slow leak will constantly re-introduce contaminants from the outside air, negating the benefits of careful purging.
Outgassing: The Hidden Contaminant Source
As the furnace heats up, the surfaces inside the tube—and the sample itself—can release trapped water vapor and other gases. This "outgassing" can re-contaminate your carefully purified atmosphere. For extremely sensitive processes, a preliminary "bake-out" under vacuum may be required to drive off these volatiles before introducing the process gas.
Defining Your Purging Strategy
The number of purge cycles you perform should be dictated by the sensitivity of your experimental materials to contamination.
- If your primary focus is extreme purity (e.g., processing oxygen-sensitive materials or growing single crystals): Perform a minimum of 3-5 purge cycles to reduce contaminants to trace levels.
- If your primary focus is general inert processing (e.g., standard annealing): One to two thorough purge cycles are often sufficient to prevent significant oxidation or unwanted reactions.
- If your primary focus is simply preventing gross oxidation: A single, robust evacuation followed by a backfill to a positive pressure will likely meet your needs.
By mastering the vacuum purge cycle, you gain precise control over your experimental environment, ensuring the integrity and repeatability of your results.
Summary Table:
| Purge Goal | Recommended Number of Cycles | Key Action |
|---|---|---|
| Extreme Purity (e.g., for oxygen-sensitive materials) | 3-5 cycles | Reduces contaminants to trace (ppm) levels. |
| General Inert Processing (e.g., standard annealing) | 1-2 cycles | Prevents significant oxidation. |
| Prevent Gross Oxidation | 1 thorough cycle | Provides a basic protective atmosphere. |
Achieve Uncompromising Atmospheric Purity with KINTEK
Does your research demand a perfectly controlled furnace atmosphere? The vacuum purging technique is essential, but its success hinges on a furnace system with superior sealing and vacuum capabilities.
KINTEK's high-temperature tube furnaces are engineered for purity. Leveraging our exceptional R&D and in-house manufacturing, we build furnaces with robust sealing flanges and compatibility with high-performance vacuum systems to ensure your purge cycles are effective and reliable. Our strong deep customization capability allows us to tailor a furnace solution—whether a standard Tube Furnace or a sophisticated Vacuum & Atmosphere system—to your exact experimental requirements.
Let us help you eliminate contamination and ensure the integrity of your results.
Contact our experts today to discuss your specific needs and discover the ideal furnace for your lab.
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What is an example of a material prepared using a tube furnace? Master Precise Material Synthesis
- Why is a tube furnace utilized for the heat treatment of S/C composite cathode materials? Optimize Battery Stability
- How does a vertical tube furnace achieve precise temperature control? Unlock Superior Thermal Stability for Your Lab
- What are the key operational considerations when using a lab tube furnace? Master Temperature, Atmosphere & Safety
- What safety measures are essential when operating a lab tube furnace? A Guide to Preventing Accidents



















