You’ve done everything by the book. Your vacuum distillation furnace is state-of-the-art, the vacuum pressure is perfect, and the temperature profile has been meticulously calibrated. Hours later, you run the final analysis on your newly purified metal, only to find the results are disappointing. The purity is nowhere near the target, and worse, you’ve introduced a new, unexpected contaminant. It’s a frustrating scenario that sends researchers and engineers back to the drawing board, questioning every parameter except the one most likely to be the culprit: the crucible itself.
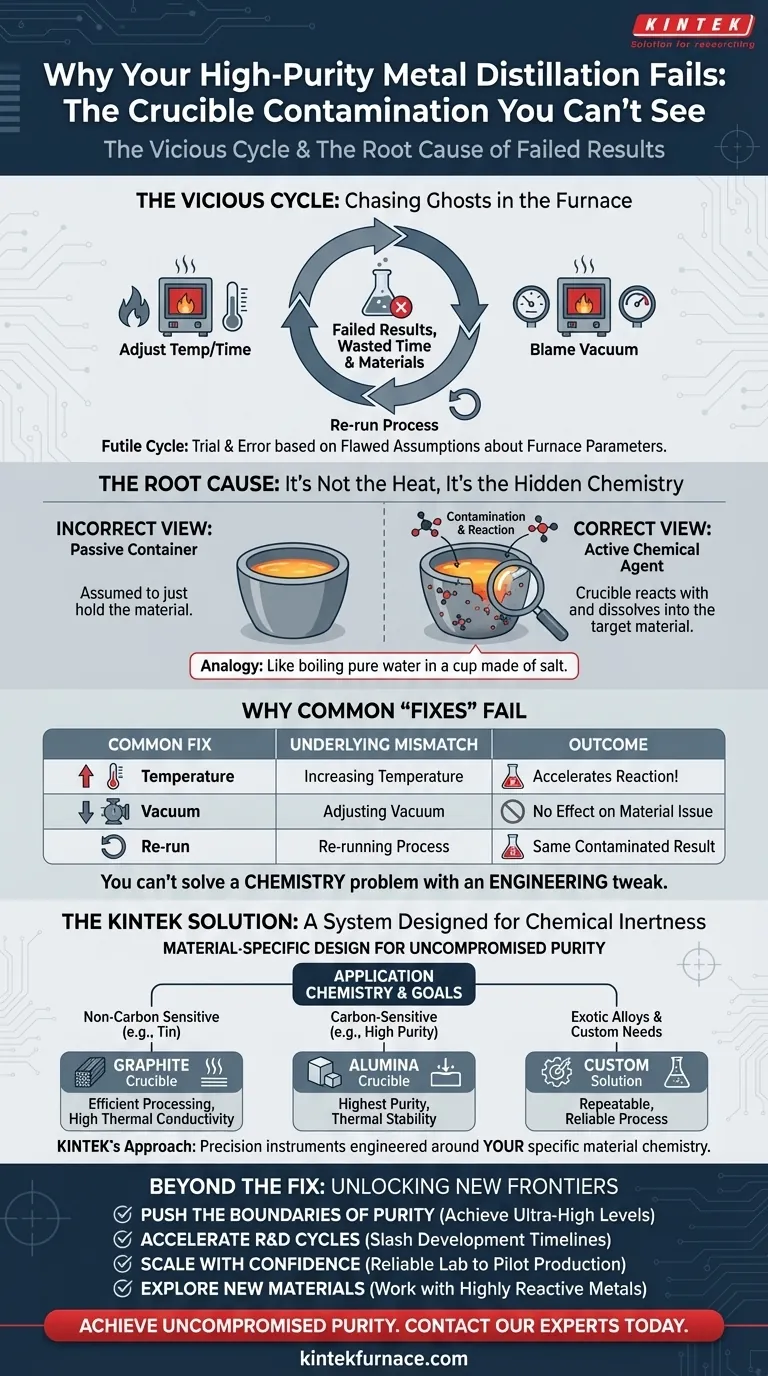
The Vicious Cycle: Chasing Ghosts in the Furnace
This problem is far more common than many labs care to admit. When faced with a contaminated product, the typical response is a frustrating cycle of trial and error:
- "Let's adjust the temperature and time." Teams will slightly increase the heat or extend the distillation time, hoping to "burn off" more impurities.
- "Maybe the vacuum wasn't strong enough." They'll blame the pump or check for micro-leaks, spending hours on equipment diagnostics.
- "We'll just run it again." Believing it was a one-off fluke, they repeat the entire costly and time-consuming process, only to get the same inconsistent results.
These actions all stem from the same flawed assumption: that the problem lies within the furnace's operating parameters. The commercial consequences are significant. Each failed run translates directly into wasted high-value materials, squandered energy, and critical project delays. For R&D teams, it undermines the integrity of their data; for manufacturers, it jeopardizes product quality and yield.
The Root of the Problem: It's Not the Heat, It's the Hidden Chemistry
Here is the turning point: the crucible is not just a passive container. At the extreme temperatures and low pressures of a vacuum furnace, it becomes an active chemical agent. The true saboteur of your process isn't that your crucible will melt—it’s that it will react.
Think of it like trying to boil ultra-pure water in a cup made of salt. The cup holds the water perfectly, but it slowly dissolves, contaminating the very thing you want to keep clean.
This is precisely what happens inside a furnace. A crucible material might be chosen for its exceptional heat resistance, but if it has a chemical affinity for the molten metal it holds, a reaction is inevitable.
Why Common "Fixes" Fail
Understanding this fundamental principle reveals why the typical troubleshooting steps are doomed to fail:
- Increasing the temperature doesn't solve the problem; it accelerates the unwanted chemical reaction between the crucible and the metal.
- Adjusting the vacuum has no effect on the underlying material incompatibility.
- Re-running the process with the same type of crucible will, without fail, produce the same contaminated result.
The issue isn't a faulty procedure; it's a fundamental mismatch in materials science. You can't solve a chemistry problem with an engineering tweak.
The Solution Embodied: A System Designed for Chemical Inertness
To guarantee purity, you need to shift your focus from simply containing the heat to ensuring absolute chemical compatibility. The solution is not just a better crucible, but an integrated system designed around the specific chemistry of your application. The right tool must be chosen based on what it doesn't do: it must not react with, dissolve into, or otherwise contaminate your target material.
This is where KINTEK's deep specialization becomes critical. We recognize that the furnace is only one part of a complex process. Our approach is built on a foundational understanding of materials science, allowing us to design and build truly custom furnace solutions.
- For distilling metals like tin where carbon is not a concern, we can engineer a system optimized for a Graphite crucible, maximizing its superior thermal conductivity for faster, more efficient processing.
- For achieving the highest purity with carbon-sensitive materials, we design around an Alumina crucible, ensuring the entire system compensates for its different thermal properties to maintain stability and prevent thermal shock.
- For unique applications with exotic alloys, our R&D and in-house manufacturing capabilities allow us to develop fully custom solutions, selecting or creating the ideal materials to ensure your process is not just successful, but repeatable and reliable.
Our products are the embodiment of this philosophy—they are not just furnaces, but precision instruments designed to control the hostile high-temperature environment so your chemical process can succeed without interference.
Beyond the Fix: Unlocking New Frontiers in Materials Science
When you eliminate the frustrating variable of crucible contamination, you stop wasting time troubleshooting and start accelerating innovation. A reliable, predictable distillation process unlocks powerful new possibilities:
- Push the Boundaries of Purity: Instead of just meeting specs, you can now confidently pursue ultra-high purity levels previously out of reach.
- Accelerate R&D Cycles: Repeatable, trustworthy results mean you can move faster from hypothesis to validated discovery, slashing development timelines for new materials and alloys.
- Scale with Confidence: A process that is reliable at the lab scale can be scaled to pilot production with far greater confidence, reducing risk and cost.
- Explore New Materials: You can now confidently work with highly reactive or sensitive metals that were previously too challenging to purify.
Solving the crucible problem isn't just about fixing a failed experiment. It's about building a foundation of reliability that allows your organization to explore, innovate, and lead in your field.
Your project has unique chemical and thermal demands that off-the-shelf solutions simply cannot address. Our team combines furnace engineering with deep materials science expertise to deliver a system tailored to your specific goals, ensuring you achieve uncompromised purity and predictable results every time. To discuss how we can solve your most challenging high-temperature application, Contact Our Experts.
Visual Guide
Related Products
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
Related Articles
- Cracked Zirconia, Inconsistent Shades: The Real Reason Your Sintering Furnace Is Failing You
- Beyond the Limits of Quartz: A Scientist's Guide to Corundum Tube Furnaces
- Why Your Sintered Parts Fail: It’s Not Just About Heat, But Pressure and Purity
- The Hidden Saboteur in Your High-Temperature Furnace: Why Your Melts Are Inconsistent—And How to Fix It
- The Microwave Sintering Trap: Why the 'Best' Furnace Might Be the Wrong Choice for Your Lab




















