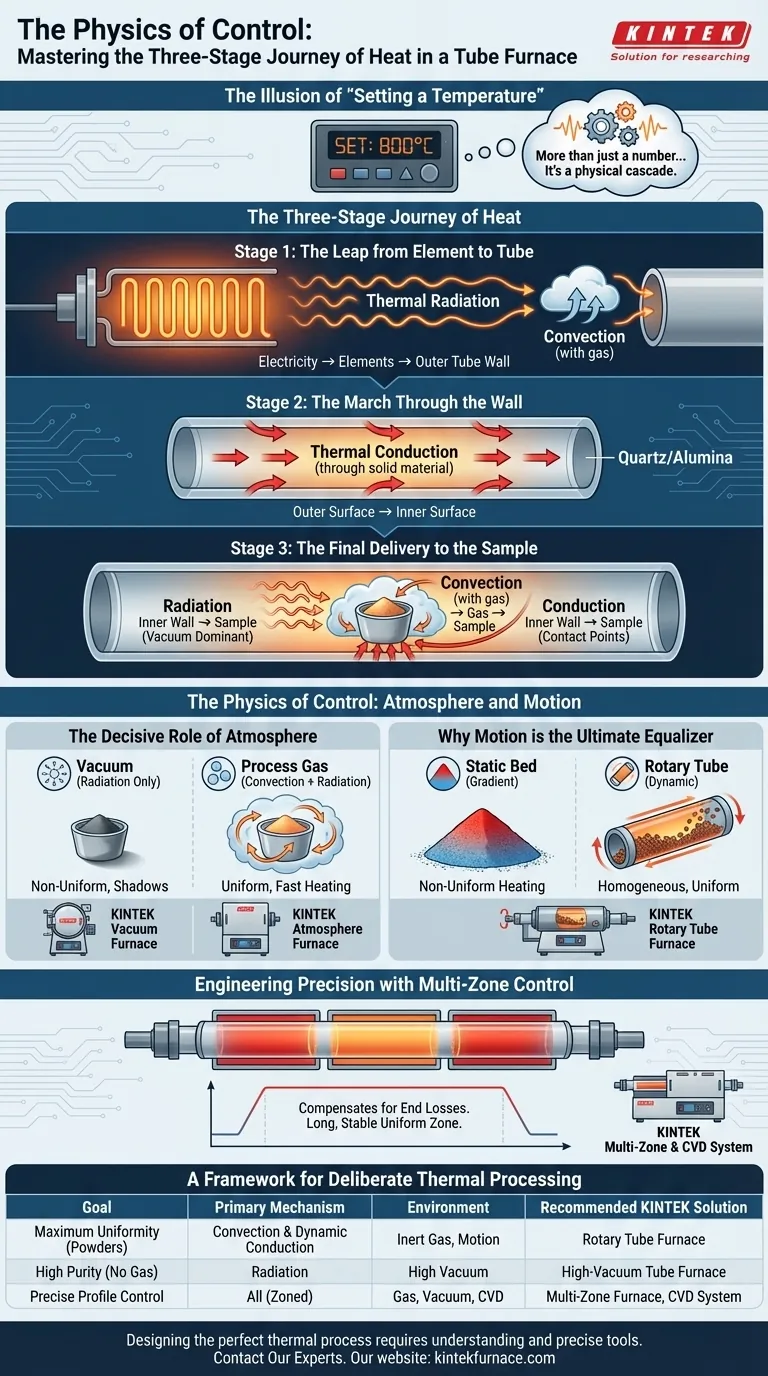
The Illusion of "Setting a Temperature"
In materials science and laboratory research, we often talk about "setting a temperature." We dial in 800°C or 1200°C and trust the machine to deliver it.
But this trust often masks a complex reality. A furnace controller displaying a setpoint doesn't guarantee your sample is at that temperature, nor that it's heating uniformly. The number on the screen is an outcome, not the process itself.
The real process is a physical journey—a cascade of energy transfer. Understanding this journey is the difference between simply running an experiment and truly controlling it.
The Three-Stage Journey of Heat
Heat transfer in a tube furnace isn't a single event. It's a sequence of handoffs, with the energy changing its mode of transport as it moves from the source to your material.
Stage 1: The Leap from Element to Tube
The journey begins with the heating elements. As electricity energizes them, they glow, releasing their energy primarily as thermal radiation. This is a non-contact transfer, an invisible wave of energy crossing the gap to the outer wall of the process tube.
If gas is present in this space, convection joins in, with circulating hot gas helping to ferry energy to the tube.
Stage 2: The March Through the Wall
Once the energy arrives at the outer surface of the tube (often made of quartz or alumina), it must pass through the solid wall. This is a job for thermal conduction.
Like a wave of vibrations passed from one molecule to the next, the heat energy marches methodically from the hotter outer surface to the cooler inner surface. The material and thickness of the tube dictate the speed of this march.
Stage 3: The Final Delivery to the Sample
This is the most critical and variable stage. The hot inner wall of the tube now becomes the new heat source for your sample, using a combination of all three mechanisms.
- Radiation: The inner wall bathes the sample's surface in thermal radiation. In a vacuum, this is the dominant—and often only—way heat can reach it.
- Convection: If a process gas is used, it heats upon contact with the wall and circulates, transferring energy to every exposed surface of your sample.
- Conduction: Where your sample physically touches the tube, heat transfers directly. It's efficient but limited to the points of contact.
The Physics of Control: Atmosphere and Motion
Your ability to control an experiment hinges on which of these transfer mechanisms you choose to amplify or suppress. This is not a limitation; it is your primary lever of control.
The Decisive Role of Atmosphere
The environment inside the tube is the single most important factor in the final stage of heat transfer.
In a vacuum, you eliminate convection entirely. Heat transfer relies on line-of-sight radiation. For a sample with a complex shape or for powders, this can create "shadows"—cooler regions that heat up much slower than the surfaces directly facing the hot tube wall. This is a common source of non-uniformity.
By introducing a process gas, you turn on convection. The circulating gas acts as an enveloping heat-transfer medium, reaching areas radiation cannot. It promotes uniformity and can drastically speed up heating. This is why KINTEK's specialized Vacuum & Atmosphere Furnaces are so critical; they give researchers precise command over this fundamental physical variable.
Why Motion is the Ultimate Equalizer
Consider heating a static bed of powder. The bottom layer heats efficiently through conduction, but the top layers rely on much slower radiation and convection through the powder itself. The result is a significant temperature gradient.
The most elegant solution to this problem is motion. A Rotary Tube Furnace fundamentally changes the physics of the process. By constantly tumbling the material, it ensures every particle is systematically exposed to all three heat transfer modes:
- Direct contact with the hot wall (conduction).
- The hot atmosphere (convection).
- The radiant energy from the tube walls.
This turns a static heating problem into a dynamic, homogenous one, delivering unparalleled uniformity that is physically impossible to achieve in a static tube.
Engineering Precision with Multi-Zone Control
Even with a perfect atmosphere, heat naturally escapes from the ends of the tube. This creates a temperature drop, shrinking your usable uniform heating zone.
Multi-zone furnaces solve this by creating independent heating zones along the tube's length. This allows you to engineer a temperature profile, compensating for end losses and creating a long, stable, and exceptionally uniform thermal environment. For sensitive processes like Chemical Vapor Deposition (CVD), where even minor temperature fluctuations can ruin film quality, this level of control—as found in KINTEK's CVD/PECVD systems—is not a luxury, it's a necessity.
A Framework for Deliberate Thermal Processing
Your experimental goal should dictate your furnace configuration. By understanding the physics, you can select the right tool for the job.
| Goal | Primary Mechanism to Exploit | Environment | Recommended KINTEK Solution |
|---|---|---|---|
| Maximum Uniformity (Powders) | Convection & Dynamic Conduction | Inert Gas, Motion | Rotary Tube Furnace |
| High Purity (No Gas) | Radiation | High Vacuum | High-Vacuum Tube Furnace |
| Precise Profile Control | All (Zoned) | Gas, Vacuum, CVD | Multi-Zone Furnace, CVD System |
Designing the perfect thermal process requires a deep understanding of physics and access to precisely engineered tools. If you're ready to move beyond just setting a temperature and start mastering your results, Contact Our Experts.
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
Related Articles
- The Unsung Hero of the Lab: The Deliberate Design of the Single-Zone Split Tube Furnace
- Your Furnace Isn't Just a Heater: Why 'Good Enough' Equipment Is Sabotaging Your Advanced Materials Research
- The Geometry of Progress: Why the 70mm Tube Furnace is a Laboratory Cornerstone
- Why Your High-Temperature Furnace Fails: The Hidden Culprit Beyond the Cracked Tube
- Beyond Heat: The Unseen Power of Environmental Control in Tube Furnaces




















