Operating Molybdenum Disilicide (MoSi2) heating elements for extended periods between 400°C and 700°C is prohibited because it triggers a catastrophic form of low-temperature oxidation. This process, often called "pest oxidation," causes the element to swell, crack, and disintegrate into a powder, leading to rapid and complete failure.
The core issue is not simple wear and tear; it's a specific chemical attack. In the 400-700°C window, the material's protective self-healing mechanism does not activate, leaving it vulnerable to a destructive oxidation process that mechanically destroys it from within.

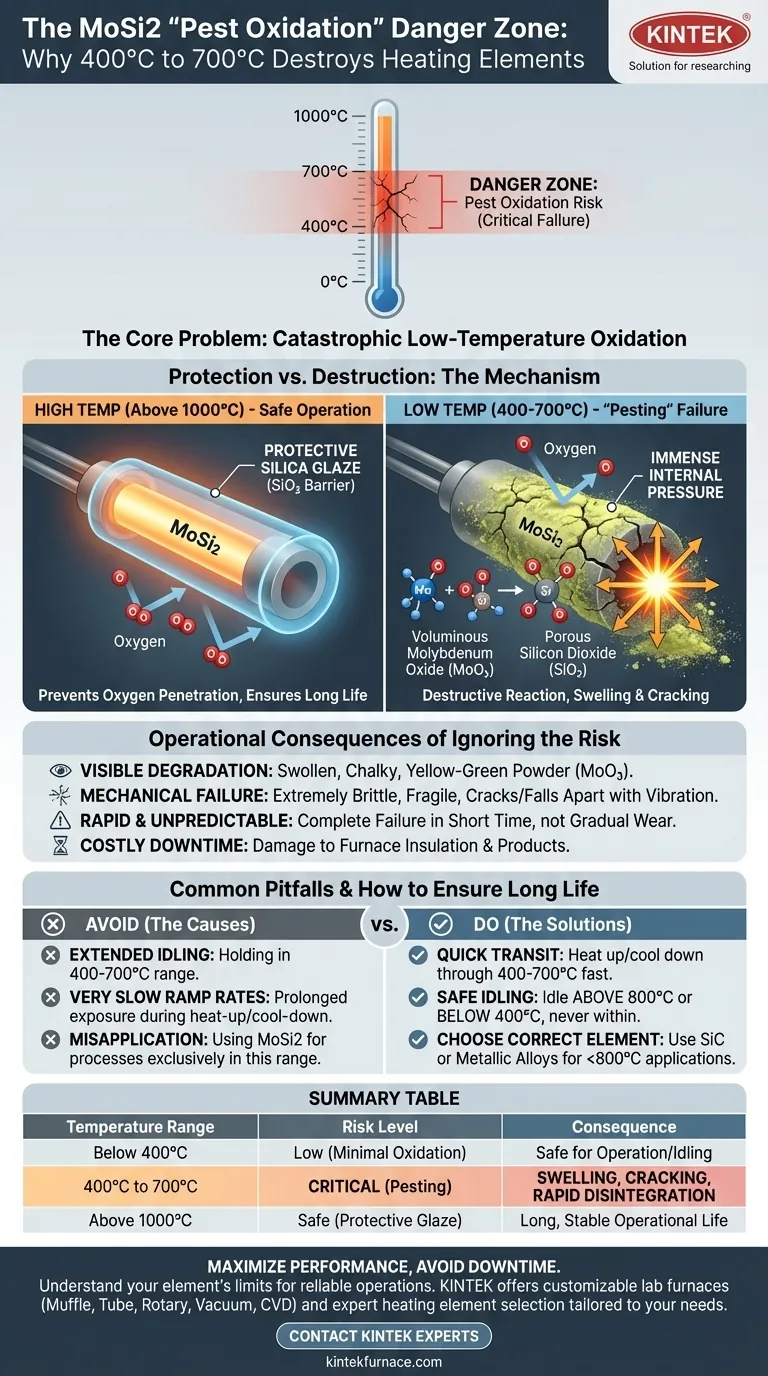
The Science Behind "Pest" Oxidation
To understand why this temperature range is so destructive, you must first understand how MoSi2 elements are designed to protect themselves at high temperatures. The problem arises when this protection is absent.
The Protective Glaze That Isn't There Yet
At high temperatures, typically above 1000°C, MoSi2 elements are exceptionally durable. They react with oxygen in the air to form a thin, non-porous layer of silica glass (SiO₂) on their surface.
This glassy layer acts as a protective barrier, preventing further oxygen from reaching the underlying MoSi2 material and ensuring a long, stable operational life.
The Vulnerable Low-Temperature Window
The temperature range of 400°C to 700°C is a critical weakness. In this window, the temperature is high enough for oxygen to react aggressively with the element but too low to form the protective, fluid silica glass layer.
Instead of a smooth glaze, a porous and non-protective mix of oxides forms.
The Mechanism of Destruction
This low-temperature process allows oxygen to penetrate the element's porous structure. It reacts with both molybdenum and silicon simultaneously.
The reaction forms solid molybdenum oxide (MoO₃) and silicon dioxide (SiO₂). The formation of these oxides, particularly the voluminous MoO₃, creates immense internal pressure. This pressure causes the element to swell and crack, exposing fresh MoSi2 material to more oxygen.
This creates a destructive feedback loop. The element essentially self-destructs, crumbling into a fine powder, which is why the phenomenon is known as "pesting."
Understanding the Operational Consequences
Ignoring this limitation leads to predictable and costly failures that go beyond simple element burnout.
Visible Degradation
An element suffering from pest oxidation will not look like a clean burnout. It may appear swollen, chalky, or covered in a yellow-greenish powder (the molybdenum oxides).
Mechanical Failure
The element becomes extremely brittle and fragile. Even minor vibrations can cause it to crack or completely fall apart, potentially damaging furnace insulation or the product being heated.
Rapid and Unpredictable Failure
Unlike high-temperature wear, which is often gradual, pesting can cause a complete failure in a very short amount of time if the element is held within the critical temperature range.
Common Pitfalls to Avoid
Most failures related to pesting are caused by operational errors rather than defects in the element itself. Understanding these common mistakes is crucial for prevention.
Extended Idling
The most common cause of pesting is allowing a furnace to idle for many hours or days within the 400-700°C range. This gives the destructive oxidation process the time it needs to cause significant damage.
Very Slow Heat-Up or Cool-Down Cycles
While all elements must pass through this temperature range, extremely slow ramp rates increase the total time spent in the danger zone. It's the prolonged exposure, not the passage itself, that causes the issue.
Misapplication in Low-Temperature Processes
Using MoSi2 elements in an application that only operates between 400°C and 700°C is a fundamental design error. These elements are designed for high-temperature work and are unsuitable for continuous low-temperature processes.
How to Ensure Long Element Life
You can completely avoid pest oxidation by respecting the element's chemical properties and operating it correctly.
- If your process requires high temperatures (above 1000°C): Program your controller to heat up and cool down through the 400-700°C range as quickly as is safely possible.
- If your process involves frequent idling: Set your idle temperature to be above the pesting range (e.g., 800°C) or below it, but never within it.
- If your process operates exclusively below 800°C: MoSi2 is the wrong heating element for your application; you should use an alternative like Silicon Carbide (SiC) or a metallic alloy element.
Understanding this critical temperature window is the key to unlocking the exceptional performance and lifespan of your MoSi2 heating elements.
Summary Table:
| Temperature Range | Risk Level | Key Process | Consequence |
|---|---|---|---|
| Below 400°C | Low | Minimal Oxidation | Safe for operation/idling |
| 400°C to 700°C | Critical (Pesting) | Non-protective oxidation, internal pressure | Swelling, cracking, rapid disintegration |
| Above 1000°C | Safe | Protective SiO₂ layer forms | Long, stable operational life |
Maximize your furnace's performance and avoid costly downtime.
Understanding the specific limitations of heating elements like MoSi2 is crucial for reliable lab operations. Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, CVD systems, and other lab high-temp furnaces, all customizable for unique needs.
Our team can help you select the right furnace and heating elements for your specific temperature profile, ensuring efficiency and longevity. Contact us today to discuss your application and get a solution tailored for your success.
Visual Guide
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the purpose of setting a mid-temperature dwell stage? Eliminate Defects in Vacuum Sintering
- What is the role of vacuum pumps in a vacuum heat treatment furnace? Unlock Superior Metallurgy with Controlled Environments
- Why is a vacuum environment essential for sintering Titanium? Ensure High Purity and Eliminate Brittleness
- How does the ultra-low oxygen environment of vacuum sintering affect titanium composites? Unlock Advanced Phase Control
- What is the purpose of a 1400°C heat treatment for porous tungsten? Essential Steps for Structural Reinforcement



















