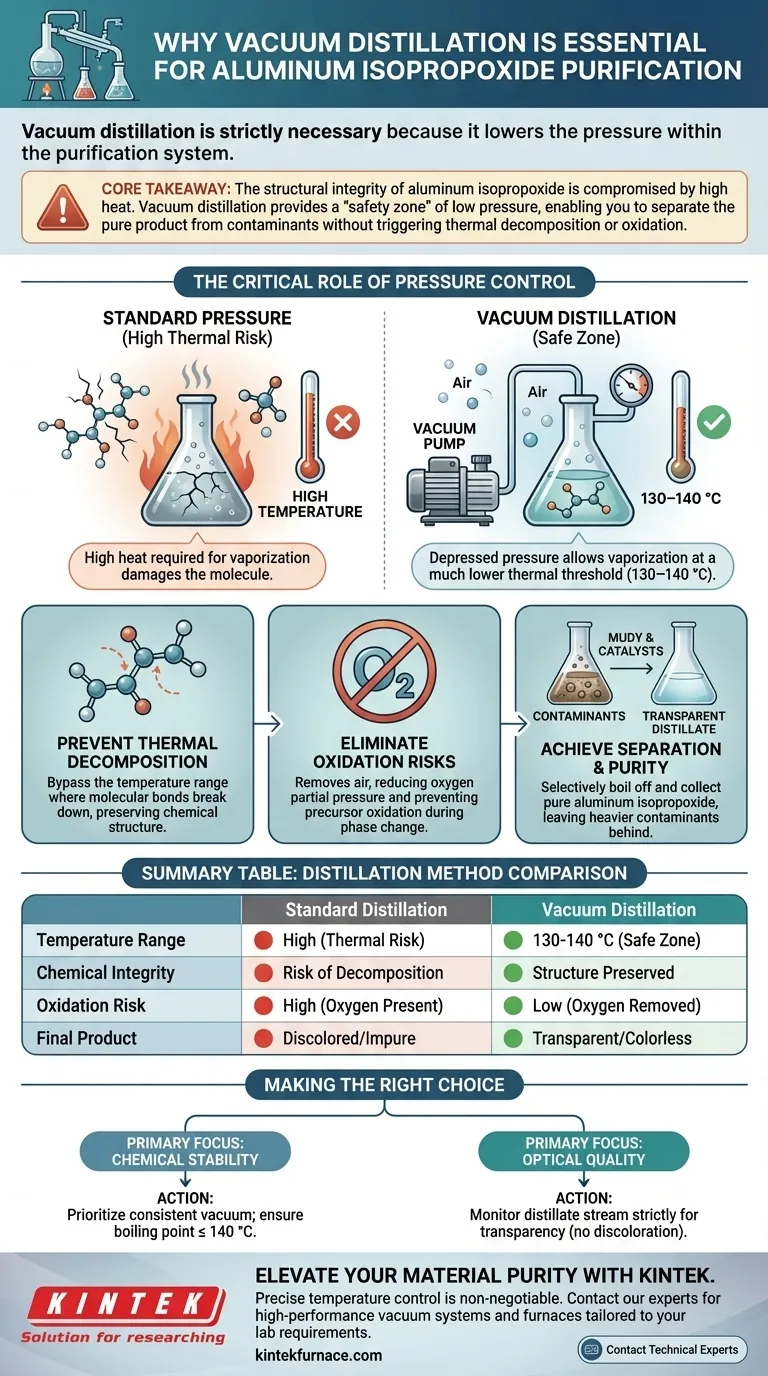
Vacuum distillation is strictly necessary because it lowers the pressure within the purification system, which significantly reduces the boiling point of aluminum isopropoxide. This allows the substance to be distilled and collected at a much lower temperature range—specifically 130–140 °C—rather than the higher temperatures required at standard atmospheric pressure.
Core Takeaway: The structural integrity of aluminum isopropoxide is compromised by high heat. Vacuum distillation provides a "safety zone" of low pressure, enabling you to separate the pure product from contaminants without triggering thermal decomposition or oxidation.
The Critical Role of Pressure Control
Lowering the Boiling Point
Under standard atmospheric pressure, the heat required to vaporize aluminum isopropoxide is high enough to damage the molecule.
Vacuum equipment artificially creates a low-pressure environment. This depression allows the liquid to transition into a vapor phase at a much lower thermal threshold, specifically between 130 °C and 140 °C.
Preventing Thermal Decomposition
High-purity precursors are often thermally unstable. If you attempt to distill them at their natural (high) boiling points, the molecular bonds may break down.
By operating under vacuum, you bypass the temperature range where thermal decomposition occurs, preserving the chemical structure of the colloid.
Eliminating Oxidation Risks
Heat accelerates oxidation, which compromises the quality of the final material.
Vacuum distillation removes air from the system, reducing the partial pressure of oxygen. This dual action of lower temperature and oxygen removal prevents the precursors from oxidizing during the phase change.
Achieving Separation and Purity
Isolating the Target Material
The primary goal of this process is to separate aluminum isopropoxide from a mixture that includes catalysts and impurities.
Because these components have different volatilities, the precise temperature control enabled by the vacuum allows you to selectively boil off and collect the aluminum isopropoxide while leaving heavier contaminants behind.
Visual Indicators of Success
The effectiveness of this vacuum-controlled separation is often visible in the physical properties of the distillate.
When the process is managed correctly within the 130–140 °C range, the result is a transparent, colorless liquid, indicating that catalysts and other color-contaminating impurities have been successfully removed.
Understanding the Trade-offs
Equipment Complexity vs. Purity
While vacuum distillation guarantees purity, it introduces mechanical complexity. You must maintain a sealed system to hold the vacuum constant.
Any fluctuation in pressure will immediately alter the boiling point. If the pressure rises (vacuum leak), the required temperature rises, bringing you back into the danger zone of decomposition.
Process Speed vs. Control
Vacuum distillation is rarely the fastest method of separation, but it is the most controlled.
Attempting to rush the process by increasing heat—rather than relying on pressure reduction—defeats the purpose of the equipment and risks degrading the entire batch.
Making the Right Choice for Your Project
To ensure the successful purification of aluminum isopropoxide, align your process parameters with your specific quality goals:
- If your primary focus is Chemical Stability: Prioritize maintaining a consistent vacuum level to ensure the boiling point never exceeds 140 °C.
- If your primary focus is Optical Quality: Monitor the distillate stream strictly for transparency; any discoloration suggests the temperature is too high or separation is incomplete.
Summary: You generally cannot purify aluminum isopropoxide colloids without vacuum distillation because the heat required at standard pressure destroys the very material you are trying to isolate.
Summary Table:
| Feature | Standard Distillation | Vacuum Distillation |
|---|---|---|
| Temperature Range | High (Thermal Risk) | 130–140 °C (Safe Zone) |
| Chemical Integrity | Risk of Decomposition | Structure Preserved |
| Oxidation Risk | High (Oxygen Present) | Low (Oxygen Removed) |
| Final Product | Discolored/Impure | Transparent/Colorless |
Elevate Your Material Purity with KINTEK
Precise temperature control is non-negotiable when handling sensitive precursors like aluminum isopropoxide. Backed by expert R&D and manufacturing, KINTEK offers high-performance vacuum systems, Muffle, Tube, Rotary, and CVD furnaces tailored for your most demanding lab requirements. Whether you need a standard setup or a fully customizable high-temperature solution, our equipment ensures your research remains free from thermal decomposition and contamination.
Ready to optimize your purification process? Contact our technical experts today to find the perfect thermal solution for your unique needs.
Visual Guide
References
- Shuang Zheng, Huanyu Zhao. Green Synthesis and Particle Size Control of High-Purity Alumina Based on Hydrolysis of Alkyl Aluminum. DOI: 10.3390/ma18092100
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Magnesium Extraction and Purification Condensing Tube Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What types of heat treatment processes are supported by vacuum furnaces? Achieve Superior Metallurgical Control
- Why is annealing niobium thin films at 600°C–800°C critical? Optimize Superconducting Performance Today
- Why is a vacuum drying oven set to 70 °C for g-C3N4/Bi2WO6? Optimize Your Photocatalyst Post-Processing
- Why is a Teflon lining required for high-pressure autoclaves? Ensure Purity in High-Entropy Oxide Synthesis
- What are the advantages of using a vacuum drying oven in the phosphor preparation process? Achieve Higher Purity Today
- What are the applications of vacuum furnaces in powder metallurgy and metal alloys? Unlock High-Purity Material Processing
- What is the purpose of using vacuum testing equipment? Achieve 100% Casting Quality via Density Index
- How do multiple-chamber vacuum furnaces enhance productivity? Boost Throughput with Continuous Workflow



















