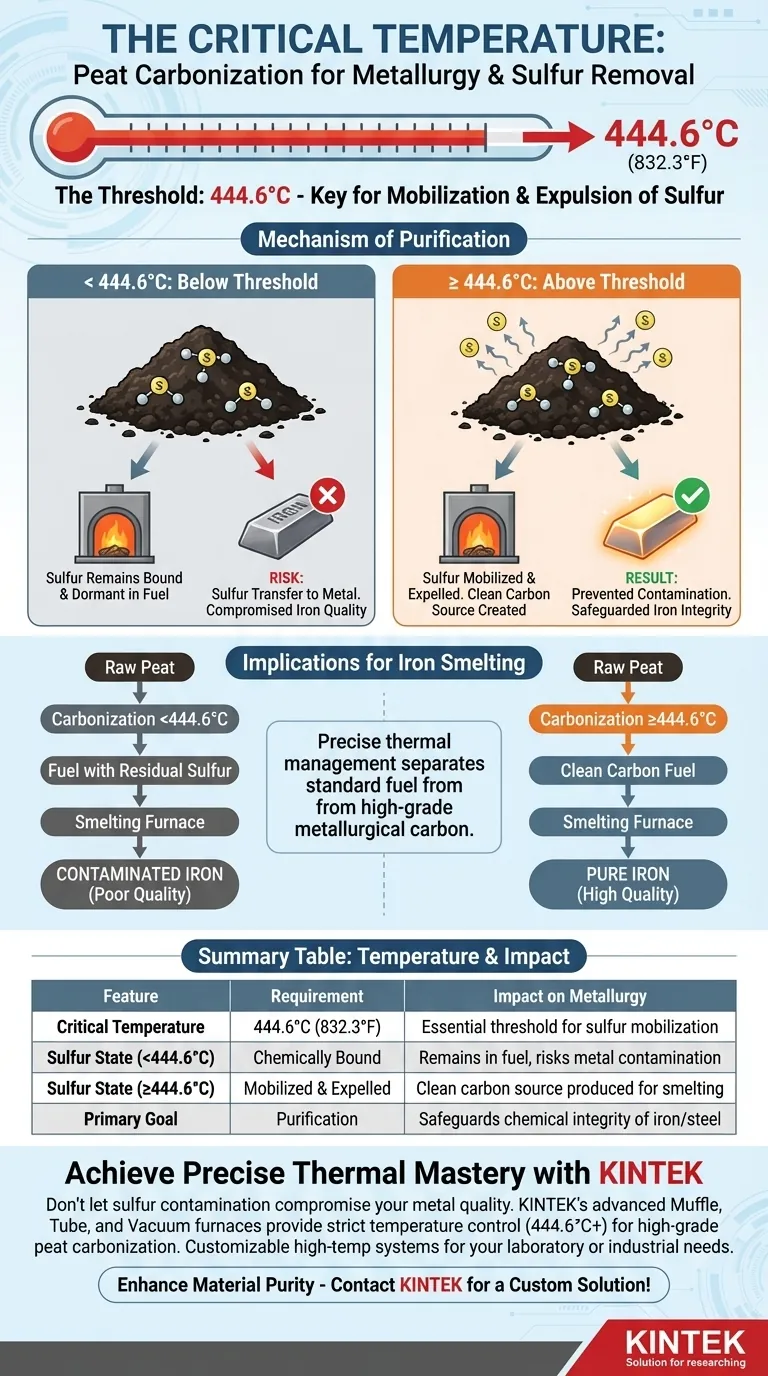
Reaching the precise temperature threshold of 444.6 degrees Celsius is critical because it triggers the effective mobilization and expulsion of sulfur from the peat. Achieving this temperature during the initial carbonization phase ensures that sulfur is driven off before the fuel is utilized, preventing it from transferring to the metal during subsequent smelting processes.
Core Takeaway For metallurgical applications, carbonizing peat is a purification process as much as it is a fuel production process. The strict requirement of reaching 444.6°C exists to eliminate sulfur early, thereby safeguarding the chemical integrity of downstream iron-smelting operations.

The Mechanism of Purification
Crossing the Thermal Threshold
The specific target of 444.6 degrees Celsius acts as a definitive tipping point for chemical changes within the peat. Below this temperature, sulfur remains chemically bound to the organic material.
Mobilization and Expulsion
Once this thermal threshold is crossed, the sulfur content is effectively mobilized. The heat drives the sulfur out of the peat mass during this initial carbonization phase. This separation is necessary to transform raw peat into a "clean" carbon source suitable for metallurgy.
Implications for Iron Smelting
Preventing Downstream Contamination
The ultimate goal of this thermal treatment is protecting the iron-smelting operation. If sulfur is not removed during carbonization, it remains dormant in the fuel.
The Risk of Transfer
When fuel containing residual sulfur is introduced to a smelting furnace, the sulfur will transfer from the fuel directly to the molten metal. This contamination compromises the quality of the iron. By removing sulfur at the carbonization stage, you eliminate this risk at the source.
Understanding the Trade-offs
Precision vs. Effort
Achieving this specific temperature requires strict process control and reliable thermal management. Failing to reach 444.6°C, even by a small margin, renders the carbonization ineffective for metallurgical purposes.
The Energy Cost of Purity
Reaching this threshold requires sustained energy input to ensure the entire mass of peat is treated. While this increases the energy cost of production, it is a necessary expense to prevent the devaluation of the final metal product.
Making the Right Choice for Your Goal
Understanding this thermal requirement allows you to categorize your fuel production based on the intended end-use.
- If your primary focus is Metallurgical Quality: You must ensure your carbonization equipment is calibrated to consistently exceed 444.6°C to guarantee maximum sulfur removal.
- If your primary focus is General Heating: You may not need to reach this specific threshold, as sulfur content is less critical for non-metallurgical thermal applications.
Precise thermal management is the defining factor that separates standard fuel from high-grade metallurgical carbon.
Summary Table:
| Feature | Requirement | Impact on Metallurgy |
|---|---|---|
| Critical Temperature | 444.6°C (832.3°F) | Essential threshold for sulfur mobilization |
| Sulfur State (<444.6°C) | Chemically Bound | Remains in fuel, risks metal contamination |
| Sulfur State (≥444.6°C) | Mobilized & Expelled | Clean carbon source produced for smelting |
| Primary Goal | Purification | Safeguards chemical integrity of iron/steel |
Achieve Precise Thermal Mastery with KINTEK
Don’t let sulfur contamination compromise your metal quality. KINTEK’s advanced Muffle, Tube, and Vacuum furnaces are engineered to provide the strict temperature control (444.6°C+) required for high-grade peat carbonization and metallurgical purification.
Backed by expert R&D and manufacturing, we offer fully customizable high-temp systems designed to meet your unique laboratory or industrial needs. Enhance your material purity today—Contact KINTEK for a custom solution!
Visual Guide
References
- Paul M. Jack. Feeling the Peat: Investigating peat charcoal as an iron smelting fuel for the Scottish Iron Age. DOI: 10.54841/hm.682
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the purpose of a microwave digestion furnace? Unlock Precise ICP-MS Results through Matrix Destruction
- Why is MFI-type zeolite (S-1) selected for H-TiO2 synthesis? Master High-Efficiency Nanoparticle Templating
- What are the objectives of melt stirring and insulation treatment during the Al-5Er-Ti master alloy preparation process?
- How does an industrial vacuum drying oven affect electrode performance? Optimize Sodium-Ion Battery Stability
- What are the energy-saving advantages of using a SHS system for tungsten carbide? Cut Energy Costs by up to 90%
- What role does a laboratory drying oven play in the post-treatment of Cu/ZIF-8 catalysts? Ensuring Structural Integrity
- Why is a precision temperature control system essential for wood carbonization? Achieve Perfect Shape Fidelity
- What is the primary purpose of high-temperature pyrolysis? Unlock Superior PFAS Removal with Enhanced Hydrophobicity



















