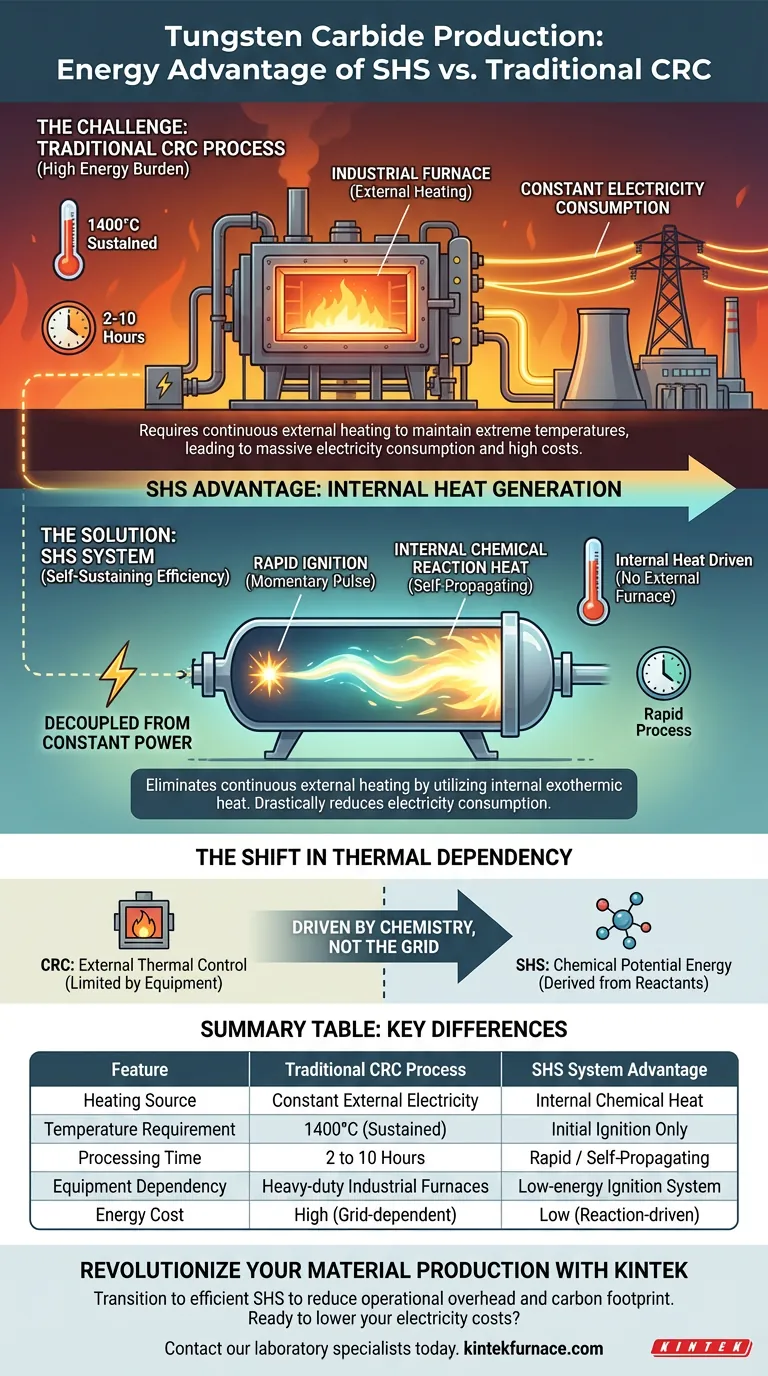
The primary energy-saving advantage of the Self-propagating High-temperature Synthesis (SHS) system lies in its ability to eliminate the requirement for continuous external heating. By utilizing the internal heat generated by the chemical reaction itself, SHS drastically reduces electricity consumption compared to traditional methods that rely on energy-intensive industrial furnaces.
Core Takeaway: The traditional Calcination-Reduction-Carburization (CRC) process is an energy burden, requiring furnaces to maintain 1400°C for up to 10 hours. In stark contrast, the SHS system requires only a momentary pulse of electricity for ignition; the process then becomes self-sustaining through internal chemical heat, effectively decoupling production from constant power consumption.
The Energy Demands of the Traditional CRC Process
To understand the efficiency of SHS, it is necessary to first examine the heavy energy load required by the traditional Calcination-Reduction-Carburization (CRC) process.
Reliance on Industrial Furnaces
The CRC method is fundamentally dependent on large-scale industrial furnaces. These units are massive consumers of electricity and must be kept active throughout the entire production cycle.
Sustained High Temperatures
The process requires maintaining an extreme temperature of 1400°C. Maintaining this thermal environment against heat loss requires a significant and constant input of energy.
Extended Processing Time
The energy consumption is compounded by the duration of the process. The furnaces must run at peak temperature for 2 to 10 hours. This prolonged exposure to high heat makes the cumulative energy cost per unit extremely high.
The SHS Advantage: Internal Heat Generation
The SHS system completely inverts the energy model used in tungsten carbide production. It shifts the source of heat from external machinery to the material itself.
The Ignition Principle
Unlike the CRC process, SHS does not require a furnace to be powered for hours. It requires only a small amount of electricity strictly for the initial ignition phase.
Self-Sustaining Reaction
Once ignited, the system generates its own internal chemical reaction heat. This exothermic energy is sufficient to drive the synthesis process to completion without further input.
Minimizing External Heating
Because the reaction propagates itself, the need for external heating is effectively minimized or eliminated after the start. This results in a production method that is not tethered to the high electricity costs associated with maintaining 1400°C environments.
The Shift in Thermal Dependency
When evaluating these systems, it is critical to understand the fundamental trade-off in how thermal energy is sourced.
External vs. Internal Reliance
The CRC process relies on external thermal control, meaning energy efficiency is limited by the insulation and efficiency of the furnace equipment.
Chemical Potential Energy
The SHS system relies on chemical potential energy. The efficiency here is derived from the formulation of the reactants rather than the power grid. This shift removes the variable of furnace run-time from the energy cost equation.
Making the Right Choice for Your Goal
The choice between these technologies often comes down to energy infrastructure and operational overhead.
- If your primary focus is reducing operational costs: The SHS system offers the most viable path by eliminating the electricity costs associated with 2-10 hour furnace cycles.
- If your primary focus is reducing infrastructure reliance: The SHS system allows you to bypass the need for heavy-duty industrial heating equipment required to maintain 1400°C.
By switching to SHS, you move from a process driven by the grid to a process driven by chemistry.
Summary Table:
| Feature | Traditional CRC Process | SHS System Advantage |
|---|---|---|
| Heating Source | Constant External Electricity | Internal Chemical Heat |
| Temperature Requirement | 1400°C (Sustained) | Initial Ignition Only |
| Processing Time | 2 to 10 Hours | Rapid / Self-Propagating |
| Equipment Dependency | Heavy-duty Industrial Furnaces | Low-energy Ignition System |
| Energy Cost | High (Grid-dependent) | Low (Reaction-driven) |
Revolutionize Your Material Production with KINTEK
Transitioning from energy-intensive CRC to an efficient SHS system can drastically reduce your operational overhead and carbon footprint. Backed by expert R&D and manufacturing, KINTEK offers a full suite of high-performance thermal solutions—including Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique synthesis needs.
Ready to lower your electricity costs and enhance production efficiency?
Contact our laboratory specialists today to find the perfect high-temperature furnace for your next project.
Visual Guide
References
- Carbon Loss and Control for WC Synthesis through a Self-propagating High-Temperature WO3-Mg-C System. DOI: 10.1007/s11665-025-10979-z
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- How are high-temperature furnaces and precision balances used for alloy oxidation kinetics? Expert Analysis
- What is the role of a precision heating system in HEA synthesis? Achieve Atomic Uniformity at 220 °C
- How is a high-stability heating stage used with a fluorescence spectrometer? Evaluate Tb3+/Ce3+ Phosphor Stability
- What is the function of a Teflon-lined autoclave in hydrothermal acid treatment? Enhance Catalyst Synthesis Efficiency
- What are the limitations of PVD coating? Overcome Challenges for Optimal Surface Engineering
- How does thermal treatment at 2400 °C improve natural graphite? Enhance Crystallinity & Electrochemical Performance
- What are the advantages of using the foil-fiber-foil method for SiC/TB8 plates? Efficient SiC/TB8 Composite Production
- What is a batch furnace and how does it operate? Master Precision Heat Treatment for Diverse Applications



















