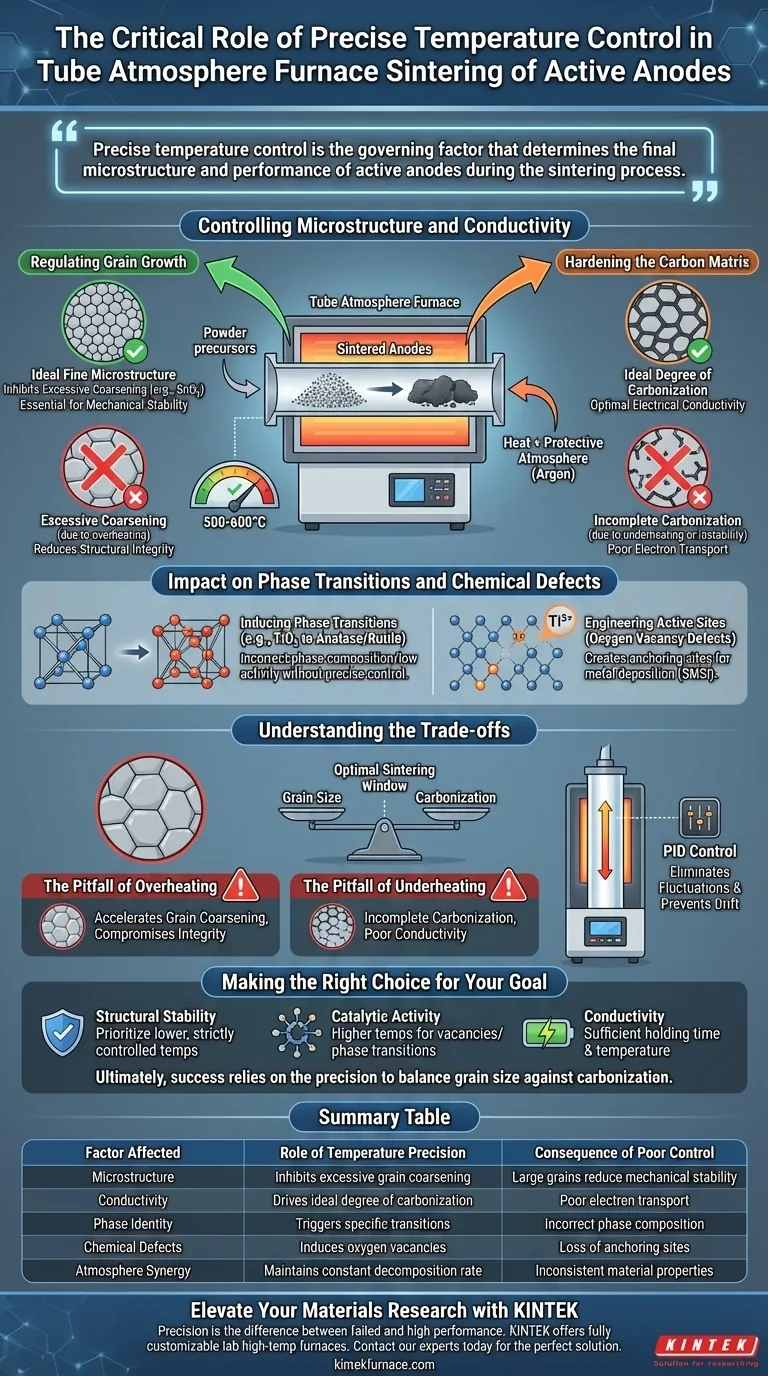
Precise temperature control is the governing factor that determines the final microstructure and performance of active anodes during the sintering process. Specifically, it regulates the decomposition rate of oxide precursors and the hardening rate of the carbon matrix, ensuring the material achieves optimal electrical conductivity without compromising its structural integrity.
The Core Insight In a tube atmosphere furnace, temperature stability prevents the excessive coarsening of oxide grains (such as SnO2) while simultaneously driving the ideal degree of carbonization in the matrix. This precise balance is the only way to manufacture anodes that possess both high electrical conductivity and long-term structural stability.

Controlling Microstructure and Conductivity
The primary challenge in sintering oxide precursors is managing two competing physical processes: grain growth and matrix hardening.
Regulating Grain Growth
During heat treatment (often between 500-600 degrees Celsius), oxide grains have a natural tendency to merge and grow.
Precise temperature regulation is critical to inhibit this process. By maintaining a strict thermal profile, you prevent the excessive coarsening of grains, such as SnO2. Keeping these grains small and uniform is essential for the mechanical stability of the final electrode.
Hardening the Carbon Matrix
Simultaneously, the furnace must supply enough energy to decompose precursors and harden the surrounding carbon matrix.
If the temperature fluctuates, the carbonization process becomes uneven. Precision ensures the matrix achieves an ideal degree of carbonization, which is directly responsible for the electrode's electrical conductivity.
The Role of Atmosphere
These processes do not happen in a vacuum, but typically under a protective atmosphere like Argon.
Temperature precision ensures the interaction between the heat and the protective atmosphere remains constant. This synergy is what allows for the exact regulation of precursor decomposition rates.
Impact on Phase Transitions and Chemical Defects
Beyond simple structure, temperature dictates the chemical identity of the anode.
Inducing Phase Transitions
Specific temperatures trigger necessary phase changes in materials.
For example, calcining at 650°C can trigger a transition in TiO2 to form a mixture of anatase and rutile phases. Without precise control, you may end up with a phase composition that lacks the desired electrochemical properties.
Engineering Active Sites
High precision allows for "defect engineering," where specific imperfections are intentionally introduced into the material.
Controlled heating in a reducing atmosphere (such as H2/Ar) induces a high concentration of oxygen vacancy defects. These defects increase the content of active ions (like Ti3+), which serve as anchoring sites for subsequent metal deposition (like Platinum) and create Strong Metal-Support Interactions (SMSI).
Understanding the Trade-offs
Achieving the perfect sinter is a balancing act. Deviating from the optimal temperature window results in specific performance penalties.
The Pitfall of Overheating
If the temperature overshoots the setpoint, grain coarsening accelerates.
While the material may be highly conductive due to complete carbonization, the large grain size reduces the active surface area and compromises the structural integrity of the anode. This often leads to electrodes that are conductive but mechanically fragile.
The Pitfall of Underheating
If the temperature is too low or unstable, the carbonization process remains incomplete.
This results in a matrix that is structurally sound (due to small grains) but suffers from poor electrical conductivity. The anode will fail to perform efficiently because the electron transport pathways are not fully established.
The Necessity of PID Control
To navigate these trade-offs, modern vertical tube furnaces utilize PID (Proportional-Integral-Derivative) algorithms.
This technology automatically adjusts heating power to eliminate fluctuations. It ensures that the heating rate, holding time, and uniformity are maintained exactly as programmed, preventing the "drift" that causes the defects mentioned above.
Making the Right Choice for Your Goal
The "perfect" temperature depends heavily on the specific oxide and the desired outcome of your anode material.
- If your primary focus is Structural Stability: Prioritize lower, strictly controlled temperatures (500-600°C) to prevent SnO2 grain coarsening and maintain a fine microstructure.
- If your primary focus is Catalytic Activity: You may need higher temperatures (e.g., 650°C) in a reducing atmosphere to induce oxygen vacancies and specific phase transitions (like in TiO2).
- If your primary focus is Conductivity: Ensure the holding time and temperature are sufficient to fully complete the carbonization of the precursor matrix.
Ultimately, the success of your sintering process relies less on the maximum temperature reached, and more on the precision with which you maintain that temperature to balance grain size against carbonization.
Summary Table:
| Factor Affected | Role of Temperature Precision | Consequence of Poor Control |
|---|---|---|
| Microstructure | Inhibits excessive grain coarsening (e.g., SnO2) | Large grains reduce mechanical stability |
| Conductivity | Drives ideal degree of carbonization in matrix | Poor electron transport or fragile structure |
| Phase Identity | Triggers specific transitions (e.g., Anatase/Rutile) | Incorrect phase composition/low activity |
| Chemical Defects | Induces oxygen vacancies and active sites | Loss of anchoring sites for metal deposition |
| Atmosphere Synergy | Maintains constant precursor decomposition rate | Inconsistent material properties |
Elevate Your Materials Research with KINTEK
Precision is the difference between a failed experiment and a high-performance anode. At KINTEK, we understand that maintaining exact thermal profiles is critical for your sintering success.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. Our lab high-temp furnaces are fully customizable to meet your unique temperature stability and atmosphere requirements, ensuring you achieve the perfect balance of microstructure and conductivity.
Ready to optimize your sintering process? Contact our experts today to find the perfect furnace solution for your laboratory.
Visual Guide
References
- Antunes Staffolani, Francesco Nobili. Tailoring the Electrochemical Performance of SnO<sub>2</sub>‐Based Anodes for Li‐Ion Batteries: Effect of Morphology and Composite Matrix. DOI: 10.1002/admt.202402058
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- Why is an inert atmosphere necessary for SPAN carbonization? Achieve High-Quality Synthesis with Precise Control
- How do high-temperature ovens and nitrogen purging systems facilitate the regeneration of activated carbon? Restore Performance
- Why is high-temperature annealing in an Air Atmosphere Furnace necessary for YAG ceramics after vacuum sintering? Achieve Optical Clarity and Mechanical Stability
- What are the structural characteristics of an atmosphere box furnace? Key Features for Controlled Environments
- How does the experimental box type atmosphere furnace contribute to energy conservation and environmental protection? Discover Sustainable Lab Solutions
- What is a retort furnace? The Ultimate Tool for Controlled Atmosphere Processing
- Why is a uniform atmosphere important in carburizing workpieces? Ensure Consistent Hardness and Prevent Failures
- How do precision quenching and air circulation furnaces optimize bi-metal HIP component heat treatment for peak performance & integrity?



















