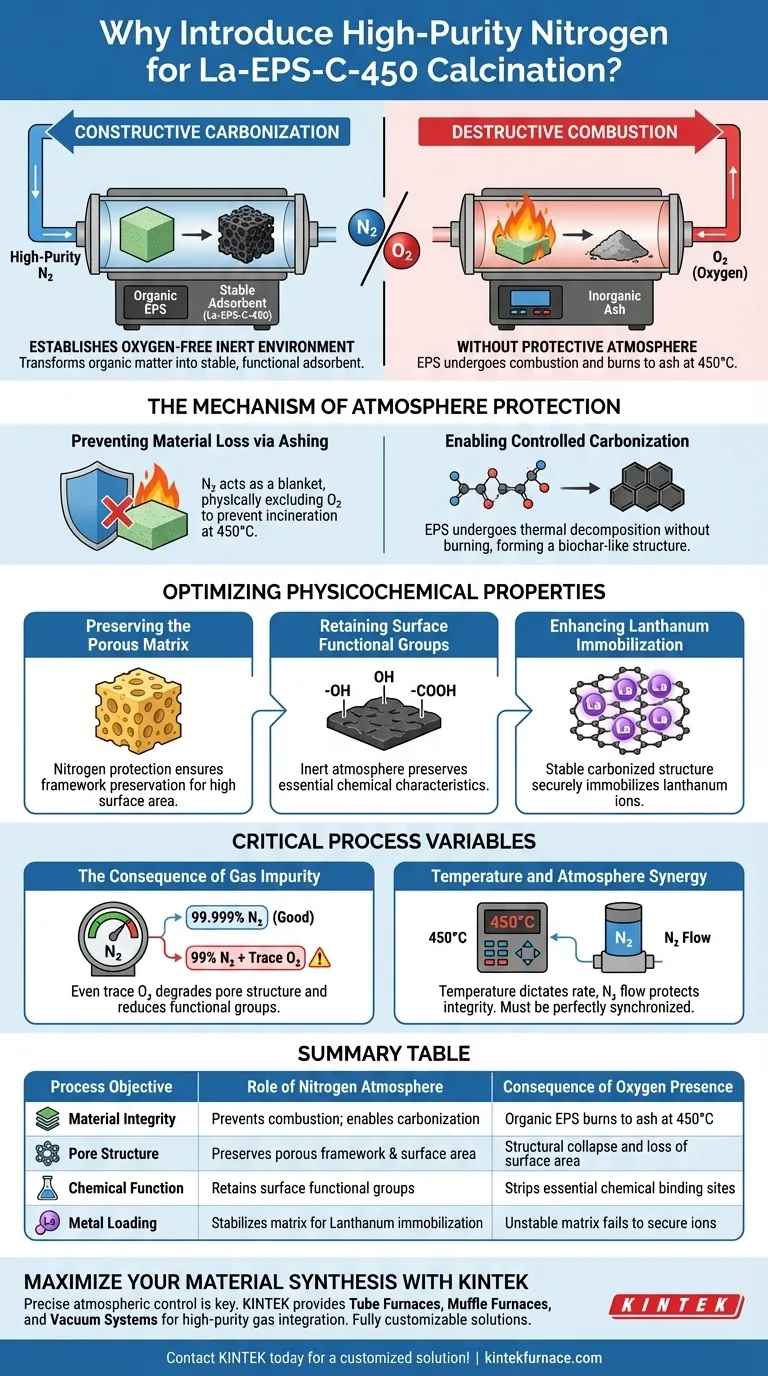
High-purity nitrogen is strictly required to establish an oxygen-free, inert environment within the tube furnace. Without this protective atmosphere, the extracellular polymeric substances (EPS) would undergo combustion and burn to ash upon reaching 450°C. Instead, the nitrogen environment forces the material to undergo carbonization, transforming organic matter into a stable, functional adsorbent rather than destroying it.
By displacing oxygen, high-purity nitrogen shifts the thermal process from destructive combustion to constructive carbonization. This ensures the formation of a rigid, porous carbon matrix capable of effectively supporting lanthanum ions.

The Mechanism of Atmosphere Protection
Preventing Material Loss via Ashing
At the high temperatures required for calcination (specifically 450°C), organic components like EPS are highly susceptible to oxidation.
If oxygen were present, the EPS would react chemically and incinerate, leaving behind only inorganic ash. High-purity nitrogen acts as a blanket, physically excluding oxygen to prevent this combustion entirely.
Enabling Controlled Carbonization
The goal of this process is not just to heat the material, but to carbonize it.
In an inert nitrogen atmosphere, the EPS undergoes thermal decomposition without burning. This process transforms the raw organic precursors into a stable, biochar-like structure, which forms the backbone of the La-EPS-C-450 adsorbent.
Optimizing Physicochemical Properties
Preserving the Porous Matrix
The effectiveness of an adsorbent is largely defined by its surface area and pore structure.
Nitrogen protection ensures that the structural framework of the EPS is preserved and evolved into a porous carbon matrix. Oxidation would collapse these pores, rendering the material ineffective for adsorption tasks.
Retaining Surface Functional Groups
The chemical identity of the adsorbent relies on specific functional groups remaining on the surface after heating.
An oxidizing environment would strip these groups away. The inert nitrogen atmosphere preserves them, allowing the final material to maintain the necessary chemical characteristics for subsequent applications.
Enhancing Lanthanum Immobilization
The interaction between the carbon matrix and the active lanthanum component is critical.
A stable, carbonized structure—achieved only through nitrogen-protected calcination—provides the necessary physical and chemical sites to securely immobilize lanthanum ions. This directly influences the stability and performance of the final composite.
Critical Process Variables
The Consequence of Gas Impurity
It is not enough to simply have nitrogen present; the purity level is paramount.
Even trace amounts of oxygen in the gas flow can initiate partial oxidation. This can degrade the quality of the pore structure and reduce the density of functional groups, leading to a sub-optimal adsorbent.
Temperature and Atmosphere Synergy
The tube furnace provides precise temperature control, but this heat is destructive without the gas flow.
The programmed temperature of 450°C dictates the rate of carbonization, while the nitrogen flow protects the material's integrity during this evolution. Both variables must be perfectly synchronized to achieve the desired material properties.
Ensuring Synthesis Success
To guarantee the quality of your La-EPS-C-450 adsorbent, prioritize the integrity of the calcination atmosphere.
- If your primary focus is Structural Stability: Ensure the nitrogen purge is thorough before heating begins to prevent early-stage oxidation of the EPS.
- If your primary focus is Chemical Performance: Verify that the nitrogen purity is high to maximize the retention of surface functional groups and Lanthanum binding sites.
The protective nitrogen atmosphere is not just a safety measure; it is an active component in engineering the material's final performance.
Summary Table:
| Process Objective | Role of Nitrogen Atmosphere | Consequence of Oxygen Presence |
|---|---|---|
| Material Integrity | Prevents combustion; enables carbonization | Organic EPS burns to ash at 450°C |
| Pore Structure | Preserves porous framework & surface area | Structural collapse and loss of surface area |
| Chemical Function | Retains surface functional groups | Strips essential chemical binding sites |
| Metal Loading | Stabilizes matrix for Lanthanum immobilization | Unstable matrix fails to secure ions |
Maximize Your Material Synthesis with KINTEK
Precise atmospheric control is the difference between advanced carbonization and total material loss. KINTEK provides industry-leading Tube Furnaces, Muffle Furnaces, and Vacuum Systems designed for high-purity gas integration and uniform thermal processing.
Backed by expert R&D and manufacturing, our systems are fully customizable to meet the rigorous demands of adsorbent development and advanced material science. Ensure the success of your next research project—Contact KINTEK today for a customized solution!
Visual Guide
References
- Yaoyao Lu, Ren‐Cun Jin. Lanthanum and Sludge Extracellular Polymeric Substances Coprecipitation-Modified Ceramic for Treating Low Phosphorus-Bearing Wastewater. DOI: 10.3390/w17081237
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- How does the inert atmosphere heat treating process work? Prevent Oxidation for Superior Material Quality
- How does a batch type controlled atmosphere furnace operate? Master Precision Heat Treatment for Superior Materials
- Why are inert atmosphere furnaces important for graphite and carbon products? Prevent Oxidation and Ensure High-Performance Results
- What industries commonly use inert atmosphere heat treating? Key Applications in Military, Automotive, and More
- How does inert atmosphere heat treating benefit aluminum? Prevent Oxide Buildup for Superior Results



















