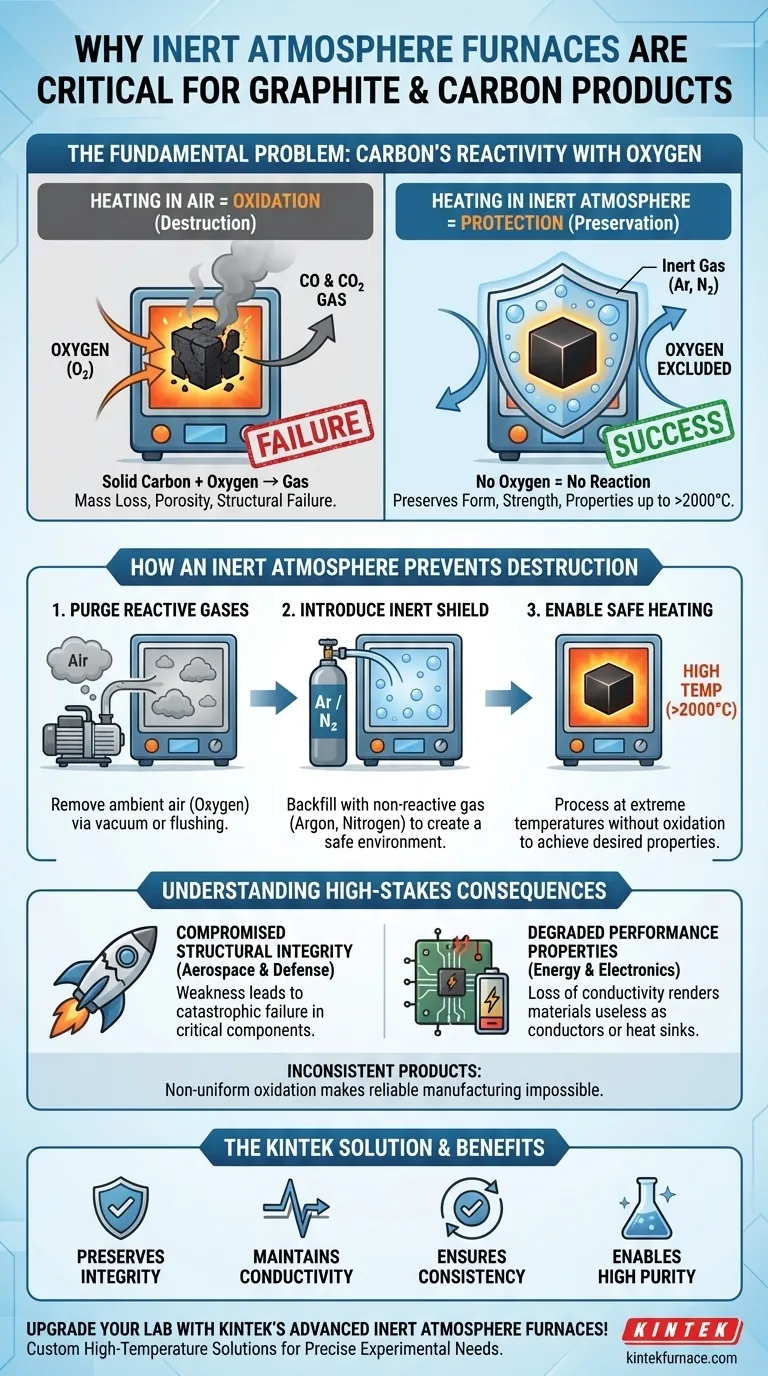
At its core, an inert atmosphere furnace is critical for processing graphite and carbon because it prevents these materials from literally burning away at high temperatures. Carbon is highly reactive with the oxygen in ambient air when heated, a process called oxidation, which converts the solid material into a gas and destroys its structural integrity and performance characteristics.
The fundamental challenge is that heat processing is necessary to achieve desired graphite properties, but this same heat makes the carbon vulnerable to destruction by oxygen. An inert atmosphere furnace resolves this conflict by replacing the reactive oxygen with a non-reactive gas, creating a safe environment for high-temperature treatment.

The Fundamental Problem: Carbon's Reactivity with Oxygen
When you process materials, you are often trying to change their structure through heat. With carbon and graphite, this presents a unique and destructive challenge.
What is Oxidation?
Oxidation is a chemical reaction between a substance and oxygen. For carbon at elevated temperatures (typically starting around 400-500°C), this reaction becomes aggressive and self-sustaining.
The carbon atoms on the surface of your product eagerly bond with oxygen molecules from the air. This is not a surface treatment; it is a destructive conversion.
The Chemical Consequence: Solid to Gas
The reaction converts solid carbon (C) into carbon monoxide (CO) and carbon dioxide (CO2) gas. This means your solid, carefully shaped part begins to disappear into the air.
This process is irreversible. You are not just discoloring the material; you are losing mass and permanently altering its composition.
The Physical Result: Material Loss and Failure
As the carbon turns into gas, the part loses mass, becomes porous, and weakens dramatically. Its dimensions change, its density drops, and its carefully engineered properties are lost.
In practice, this means a part heated in air will be structurally useless long before it reaches the temperatures required for graphitization or purification.
How an Inert Atmosphere Prevents Destruction
An inert atmosphere furnace is specifically designed to control the chemical environment, creating a shield that allows for high-temperature processing without damage.
Step 1: Purging Reactive Gases
The first step is to remove the ambient air from the furnace chamber. This is typically done by pumping the air out to create a vacuum or by flushing the chamber with a large volume of inert gas.
The goal is to eliminate the primary reactant: oxygen.
Step 2: Introducing a Non-Reactive Shield
Once the air is removed, the furnace is backfilled and pressurized with a non-reactive, or "inert," gas. The most common choices are Argon (Ar) and Nitrogen (N2).
These gases are called inert because their atoms have stable electron configurations, making them extremely reluctant to react with other elements, even at very high temperatures.
Step 3: Enabling Safe Heating
With the carbon component surrounded by a non-reactive gas, the oxidation reaction cannot occur. The oxygen simply isn't present.
This allows the material to be heated to extreme temperatures (often over 2000°C) to achieve desired properties like increased purity, crystal alignment, and conductivity, all while preserving its physical form and strength.
Understanding the High-Stakes Consequences
Failing to use an inert atmosphere isn't a minor oversight; it results in complete failure, especially in demanding industries.
Compromised Structural Integrity
For applications in aerospace or defense, where graphite components are used for rocket nozzles or heat shields, any structural weakness is catastrophic. Oxidation creates this weakness, leading to component failure.
Degraded Performance Properties
In the energy and electronics sectors, graphite is used for its excellent thermal and electrical conductivity. Oxidation destroys the crystalline structure that provides these properties, rendering the material useless as a conductor, electrode, or heat sink.
Inconsistent and Unreliable Products
Without a controlled atmosphere, oxidation occurs non-uniformly across a part's surface. This makes it impossible to manufacture products with the consistent, reliable, and predictable performance required for any high-performance application.
Making the Right Choice for Your Goal
Controlling the furnace atmosphere is a direct investment in the quality and viability of the final product. Your decision should be based on the required performance of the component.
- If your primary focus is high-performance applications (aerospace, semiconductor, energy): A high-purity inert atmosphere is absolutely non-negotiable to preserve the material's essential structural, thermal, and electrical properties.
- If your primary focus is achieving maximum purity: An inert atmosphere is required to prevent the introduction of oxygen-based impurities and to facilitate the removal of other volatile elements during heat treatment.
- If your primary focus is simply surviving high-temperature treatment: Any heating of carbon or graphite above 400-500°C mandates an inert atmosphere or vacuum simply to prevent the component from being consumed by oxidation.
Ultimately, using an inert atmosphere furnace is the enabling technology that allows carbon and graphite to be transformed into the high-performance materials modern industry depends on.
Summary Table:
| Aspect | Key Information |
|---|---|
| Problem | Carbon reacts with oxygen at high temperatures, causing oxidation that converts solid material to gas, leading to mass loss and failure. |
| Solution | Inert atmosphere furnaces use non-reactive gases like Argon or Nitrogen to prevent oxidation, enabling safe heating up to 2000°C. |
| Benefits | Preserves structural integrity, maintains thermal/electrical conductivity, ensures product consistency, and supports high-purity processing. |
| Applications | Critical in aerospace, defense, energy, and electronics industries for reliable component performance. |
Upgrade your lab's capabilities with KINTEK's advanced inert atmosphere furnaces! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with high-temperature solutions like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization ensures precise fit for your unique experimental needs, protecting your graphite and carbon products from oxidation and enhancing performance. Contact us today to discuss how we can optimize your processes and deliver reliable results!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What is the use of nitrogen in furnace? Prevent Oxidation for Superior Heat Treatment
- What is nitrogen used for in a furnace? Prevent Oxidation and Control Heat Treatment Quality
- How does an inert atmosphere prevent oxidation? Shield Materials from Oxygen Damage
- How does a chemically inert atmosphere function in a furnace? Prevent Oxidation and Ensure Material Purity
- How does the inert atmosphere heat treating process work? Prevent Oxidation for Superior Material Quality



















