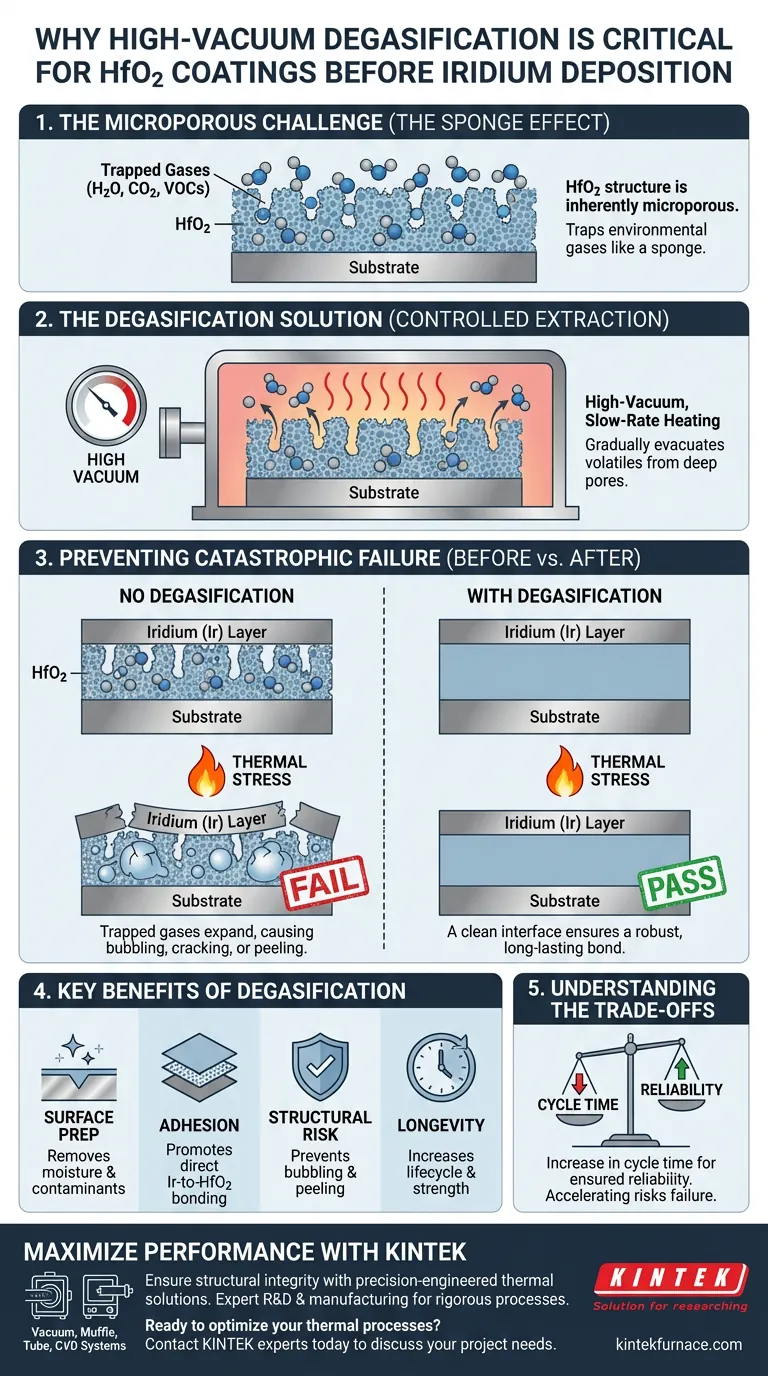
High-vacuum degasification is the defining step for coating longevity. This process is necessary because HfO2 (Hafnium Dioxide) coatings inherently possess a microporous structure that traps environmental gases. If these gases are not evacuated via slow-rate heating in a vacuum before the iridium (Ir) layer is applied, they will expand during high-temperature service, causing the iridium to bubble, crack, or peel off.
The microporous nature of HfO2 acts as a reservoir for adsorbed gases. Controlled high-vacuum degassing eliminates these gas pockets, preventing catastrophic delamination caused by thermal expansion and ensuring a robust bond between the oxide and the iridium layer.
The Challenge of Microporosity
The "Sponge" Effect
HfO2 coatings are not perfectly dense, impermeable solids. They feature a microporous structure that increases the surface area available for adsorption.
Because of this porosity, the coating easily traps gases from the surrounding environment. This often includes moisture, carbon dioxide, and volatile organic compounds (VOCs).
The Necessity of Slow Extraction
Removing these trapped volatiles is not instantaneous. It requires a high-vacuum environment combined with slow-rate heating.
This controlled approach allows gases to migrate out of the deep pores gradually. A rapid process might fail to evacuate the deepest pores, leaving residual gas pockets behind.
Preventing Catastrophic Failure
The Mechanics of Delamination
If the iridium layer is deposited without prior degassing, it effectively seals the trapped gases inside the HfO2 structure.
When the component is later exposed to high temperatures—either during subsequent processing or actual service—the trapped gases expand rapidly.
Structural Integrity Risks
The pressure generated by this thermal expansion seeks a release path. Since the iridium layer blocks the exit, the force pushes against the coating interface.
This leads to bubbling, cracking, or peeling of the iridium layer. These defects ruin the protective qualities of the coating and compromise the part's performance.
Enhancing Interlayer Adhesion
Degasification does more than just prevent cracks; it actively promotes adhesion.
By removing physical barriers like adsorbed water or organic contaminants, the iridium atoms can bond more directly with the HfO2 surface. This results in a composite coating with significantly higher interlayer strength.
Understanding the Trade-offs
Process Time vs. Reliability
The primary trade-off of high-vacuum degasification is the increase in cycle time.
Slow-rate heating processes prolong the overall manufacturing timeline. Attempting to accelerate this step to save time increases the risk of incomplete outgassing and eventual coating failure.
Equipment Complexity
This process requires specialized high-vacuum equipment capable of precise thermal control.
Standard ovens or low-vacuum systems are insufficient for removing gases trapped within micropores. This adds to the capital equipment cost and operational complexity of the coating line.
Ensuring Coating Success
To maximize the performance of your HfO2/Ir composite coatings, prioritize the preparation of the substrate interface.
- If your primary focus is coating longevity: Implement a slow-rate heating cycle to ensure gases are evacuated from the deepest micropores.
- If your primary focus is adhesion strength: Verify that the vacuum level is sufficient to remove chemically adsorbed contaminants, not just physically trapped air.
A pristine, gas-free interface is the only way to guarantee that the iridium layer remains intact under thermal stress.
Summary Table:
| Feature | Impact of High-Vacuum Degasification |
|---|---|
| Surface Preparation | Removes moisture, CO2, and VOCs from HfO2 micropores |
| Adhesion Quality | Eliminates gas barriers to promote direct Ir-to-HfO2 bonding |
| Structural Risk | Prevents bubbling, cracking, and peeling during thermal expansion |
| Process Method | Slow-rate heating in vacuum ensures deep pore evacuation |
| Coating Lifecycle | Significantly increases longevity and interlayer strength |
Maximize Your Coating Performance with KINTEK
Ensure the structural integrity of your advanced coatings with precision-engineered thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers state-of-the-art Vacuum, Muffle, and Tube systems, as well as specialized CVD systems designed to handle the rigorous demands of high-vacuum degasification and deposition processes.
Whether you are working with HfO2/Ir composites or other high-performance materials, our customizable lab high-temp furnaces provide the stable, slow-rate heating cycles essential for eliminating microporous outgassing and enhancing adhesion.
Ready to optimize your lab's thermal processes? Contact KINTEK today to discuss your unique project needs with our experts.
Visual Guide
References
- Junyu Zhu, Xuxiang Zhang. Oxidation Resistance of Ir/HfO2 Composite Coating Prepared by Chemical Vapor Deposition: Microstructure and Elemental Migration. DOI: 10.3390/coatings14060695
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering and Brazing Furnace
People Also Ask
- How is cooling typically achieved in vacuum furnaces? Master Precise Heat Treatment for Superior Results
- What advantages does laser active brazing offer compared to traditional furnace brazing? Precision Sealing Explored
- What is the purpose of using a high-vacuum drying oven? Maximize Battery Performance and Electrode Purity
- What does a vacuum furnace do? Achieve Superior Material Processing in a Pure Environment
- What are the advantages of a Vertical/Bottom Loading Vacuum Furnace? Save Space and Boost Precision
- What is the function of a vacuum chamber during the TLP bonding process? Achieve High-Purity Defect-Free Joints
- What role does a vacuum sintering furnace play in fine ceramic component production? Achieve High-Purity, Dense Ceramics
- How does optimizing the graphite base material improve the quality of cemented carbide sintering? Master Thermal Uniformity



















