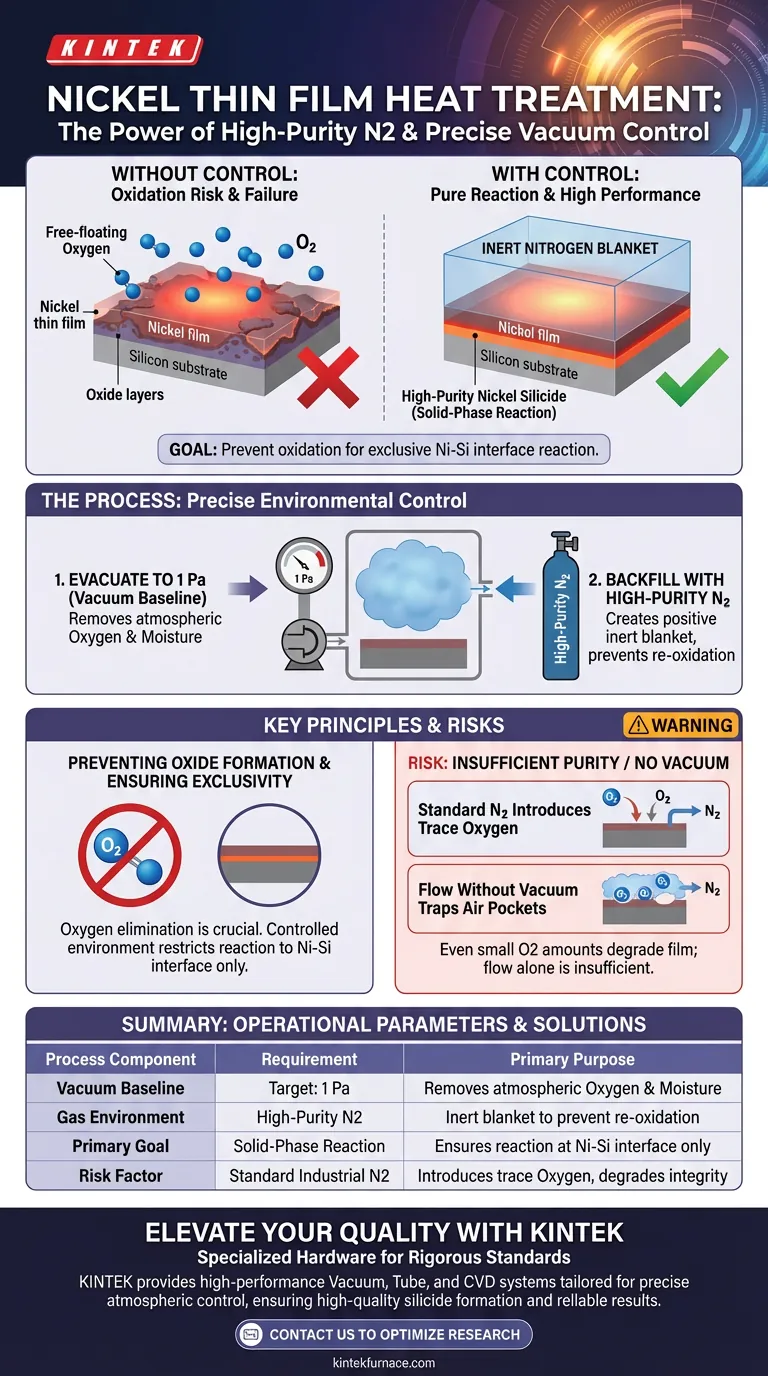
High-purity nitrogen protection and precise vacuum control are essential to prevent oxidation during heat treatment. By evacuating the chamber and backfilling it with inert gas, you eliminate oxygen interference that would otherwise degrade the nickel thin film at high temperatures. This controlled environment ensures that the chemical reaction occurs exclusively between the nickel and the silicon substrate.
The core objective of this environmental control is to disable competing chemical reactions. By removing oxygen, you force the system to undergo a solid-phase reaction only at the nickel-silicon interface, guaranteeing the formation of high-purity nickel silicide.
The Role of Oxygen Elimination
Preventing Oxide Formation
At elevated temperatures, nickel becomes highly reactive. If oxygen is present in the processing chamber, the nickel will react with it immediately.
This results in the formation of nickel oxide rather than the desired conductive material. Oxidation compromises the structural and electrical integrity of the thin film.
Ensuring Interface Exclusivity
The goal of the heat treatment is a specific solid-phase reaction. This reaction is intended to occur strictly at the interface where the nickel film meets the silicon wafer.
Oxygen acts as a barrier or a contaminant in this process. By maintaining an oxygen-free atmosphere, you ensure the reaction is limited to the Ni-Si interface, which is critical for device performance.
The Mechanism of Environmental Control
Achieving the Vacuum Baseline
Before heat is applied, the thermal processing equipment must evacuate the chamber. The target pressure is typically 1 Pa.
This step is not about creating a permanent vacuum, but about removing the baseline atmospheric air. This effectively strips the chamber of the bulk oxygen and moisture naturally present in the environment.
Backfilling with Nitrogen
Once the chamber reaches 1 Pa, it is backfilled with high-purity nitrogen. Nitrogen serves as an inert "blanket" for the film.
Because the nitrogen is high-purity, it contains negligible trace elements. It creates a positive pressure environment that prevents outside air from leaking back in while chemically ignoring the heated nickel.
Understanding the Risks and Trade-offs
The Risk of Insufficient Purity
Using standard industrial nitrogen is a common pitfall. If the nitrogen source is not high-purity, it introduces trace amounts of oxygen back into the chamber.
Even a small amount of oxygen re-introduced during backfilling can ruin the solid-phase reaction, rendering the vacuum step useless.
The Necessity of the Vacuum Step
One might assume that simply flowing nitrogen over the sample is enough. However, without the initial evacuation to 1 Pa, pockets of air remains trapped in the chamber.
Flowing nitrogen dilutes oxygen, but evacuation removes it. Relying solely on flow (purging) without vacuum is often insufficient for high-quality nickel silicide formation.
Ensuring Process Success
To ensure high-quality nickel silicide formation, focus on the following operational parameters:
- If your primary focus is Film Purity: Ensure your nitrogen source is certified high-purity to prevent trace oxidation during the backfill phase.
- If your primary focus is Process Consistency: Verify that your equipment reliably reaches the 1 Pa vacuum threshold before every single heat cycle to eliminate atmospheric variables.
Strict adherence to these environmental controls is the only way to transform a raw nickel film into a high-performance silicide contact.
Summary Table:
| Process Component | Requirement | Primary Purpose |
|---|---|---|
| Vacuum Baseline | Target: 1 Pa | Removes atmospheric oxygen and moisture |
| Gas Environment | High-Purity Nitrogen | Acts as an inert blanket to prevent re-oxidation |
| Primary Goal | Solid-Phase Reaction | Ensures reaction occurs only at Ni-Si interface |
| Risk Factor | Standard Industrial N2 | Introduces trace oxygen that degrades film integrity |
Elevate Your Thin Film Quality with KINTEK
Precise atmospheric control is the difference between a high-performance silicide contact and a failed oxidation layer. KINTEK provides the specialized hardware needed to achieve these rigorous standards. Backed by expert R&D and manufacturing, we offer high-performance Vacuum, Tube, and CVD systems designed for the specific needs of semiconductor and material science labs.
Our customizable high-temperature furnaces ensure you reach the 1 Pa threshold reliably and maintain inert gas purity throughout your cycle. Contact us today to discuss your project requirements and see how our tailored thermal solutions can optimize your research outcomes.
Visual Guide
References
- V. A. Lapitskaya, Maksim Douhal. Microstructure and Properties of Thin-Film Submicrostructures Obtained by Rapid Thermal Treatment of Nickel Films on Silicon. DOI: 10.3390/surfaces7020013
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- How are atmosphere furnaces used in the glass industry? Boost Strength and Efficiency in Glass Production
- What are some specific applications of retort furnaces? Essential for High-Purity Heat Treatment
- What is an inert oven? Protect Your Materials from Oxidation and Contamination
- How does a precision high-temperature furnace ensure the densification of MgO? Master Low-Temp Ceramic Sintering
- What is the effect of post-deposition annealing (PDA) on fluoride thin films? Optimize 2D Transistor Performance
- Why are Argon and Hydrogen utilized as the process atmosphere during plasma spraying of AlCoCrFeNi? Unlock High-Purity Coatings
- What are the advantages of a hydrogen reducing atmosphere for stainless steel MIM parts? Achieve Superior Integrity
- How do resistance furnaces and glass fiber heating mantles collaborate in distillation? Ensure Optimal Vapor Stability



















