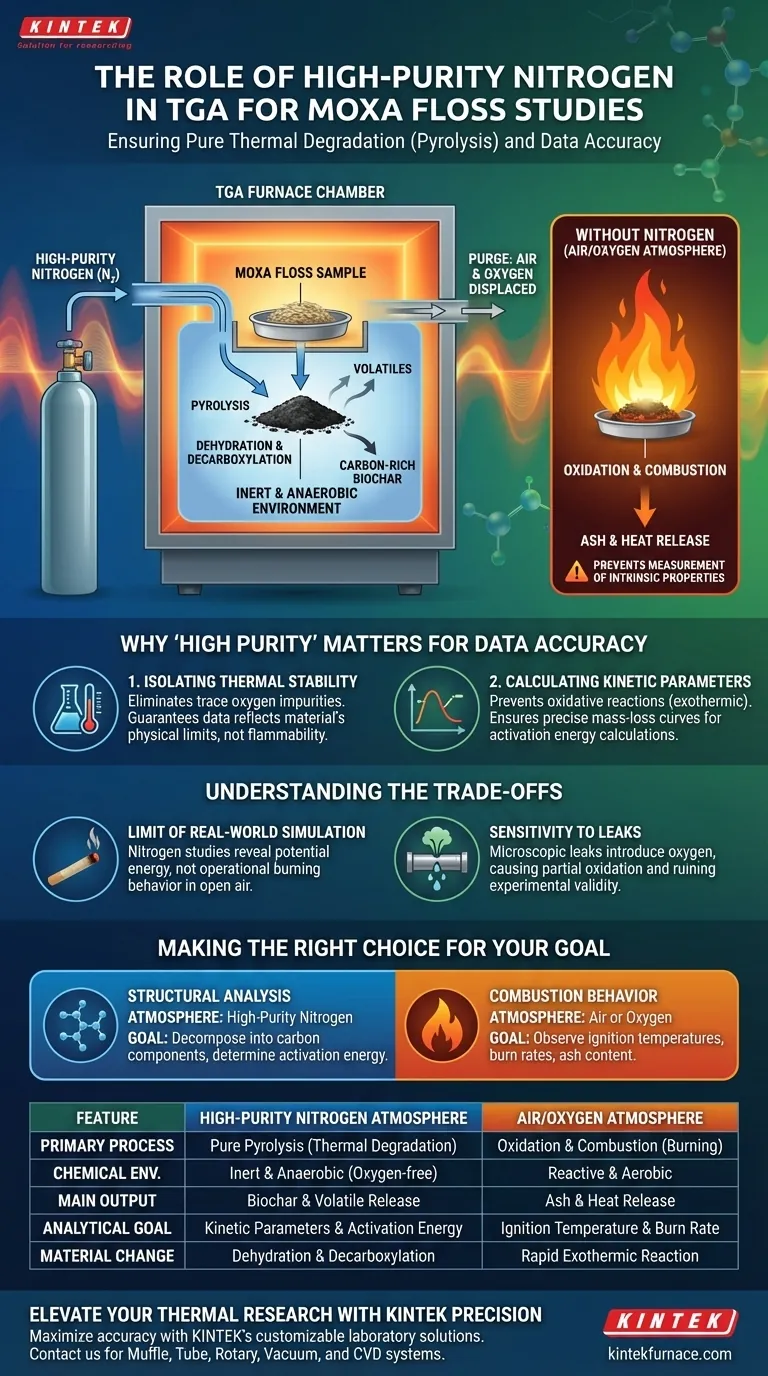
High-purity nitrogen functions as a critical isolation barrier. Its primary role in a thermogravimetric analyzer (TGA) is to purge atmospheric air from the furnace chamber, establishing a stable, inert, and anaerobic environment. This effectively prevents the moxa floss from undergoing oxidation or combustion (burning) during heating, ensuring that the changes observed are due solely to thermal degradation.
By eliminating oxygen, high-purity nitrogen forces the material to undergo pyrolysis rather than combustion. This allows researchers to measure the intrinsic thermal stability and kinetic properties of moxa floss without the chemical interference of burning.

The Mechanics of an Inert Atmosphere
Preventing Oxidation and Combustion
The presence of oxygen at high temperatures triggers immediate combustion in biomass materials like moxa floss.
High-purity nitrogen displaces the air within the furnace to create an oxygen-deficient environment.
This ensures that the mass loss observed by the TGA is not caused by the sample burning up, but by the material breaking down internally.
Ensuring Pure Pyrolysis
When heating occurs without oxygen, the process is called pyrolysis.
In this state, the complex organic structures of the moxa floss—specifically cellulose, hemicellulose, and lignin—decompose through dehydration and decarboxylation.
This leads to the release of volatiles and the formation of carbon-rich biochar, rather than simple ash.
Why "High Purity" Matters for Data Accuracy
Isolating Thermal Stability
The goal of the study is to determine the thermal stability of the moxa floss components.
If impurities or trace oxygen were present, they would react with the sample, altering the temperature points at which degradation occurs.
A strictly inert environment guarantees that the data reflects the material's physical limits, not its flammability.
Calculating Kinetic Parameters
TGA is often used to calculate reaction kinetics, such as activation energy.
These calculations rely on precise mass-loss curves derived from specific decomposition stages (moisture evaporation, volatile release, char formation).
Oxidative reactions release heat (exothermic) and alter mass unpredictably, which would invalidate these kinetic calculations.
Understanding the Trade-offs
The limit of "Real World" Simulation
While nitrogen provides analytical precision, it creates an artificial environment.
If your goal is to understand how moxa floss behaves during actual use (burning moxibustion therapy), a nitrogen atmosphere will not replicate the combustion characteristics seen in open air.
Nitrogen studies reveal potential energy and structural stability, not operational burning behavior.
Sensitivity to Leaks
The reliance on a high-purity inert gas makes the experiment highly sensitive to system integrity.
Even a microscopic leak in the tube furnace or the gas lines can introduce trace oxygen.
This "contamination" can cause partial oxidation, leading to hybrid data that represents neither pure pyrolysis nor full combustion, effectively ruining the experimental validity.
Making the Right Choice for Your Goal
The choice of atmosphere dictates the type of chemical data you will receive from the TGA.
- If your primary focus is Structural Analysis: Use High-Purity Nitrogen to decompose the material into its fundamental carbon components and determine activation energy.
- If your primary focus is Combustion Behavior: Use Air or Oxygen to observe ignition temperatures, burn rates, and ash content relevant to real-world burning.
Ultimately, the introduction of high-purity nitrogen transforms the experiment from a simple burning test into a precise dissection of the material's molecular bond strength.
Summary Table:
| Feature | High-Purity Nitrogen Atmosphere | Air/Oxygen Atmosphere |
|---|---|---|
| Primary Process | Pure Pyrolysis (Thermal Degradation) | Oxidation & Combustion (Burning) |
| Chemical Environment | Inert & Anaerobic (Oxygen-free) | Reactive & Aerobic |
| Main Output | Biochar and Volatile Release | Ash and Heat Release |
| Analytical Goal | Kinetic Parameters & Activation Energy | Ignition Temperature & Burn Rate |
| Material Change | Dehydration & Decarboxylation | Rapid Exothermic Reaction |
Elevate Your Thermal Research with KINTEK Precision
Maximize the accuracy of your material studies with high-performance laboratory solutions. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as specialized lab high-temp furnaces—all fully customizable to meet your unique research needs.
Whether you are analyzing moxa floss pyrolysis or complex material kinetics, our systems provide the stable, inert environments required for reproducible results. Contact us today to find your ideal furnace solution and see how our expertise can drive your innovation forward.
Visual Guide
References
- Yukun Feng, Zhaoyi Zhuang. Combustion Characteristics of Moxa Floss Under Nitrogen Atmosphere. DOI: 10.3390/fuels6020048
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What types of gases can a controlled atmosphere furnace handle? Master Inert and Reactive Gases for Your Lab
- How is a laboratory box furnace with a controlled atmosphere used for biomass conversion? Engineering Biochar Additives
- Why is continuous argon flow essential for biochar pyrolysis? Unlock High-Yield, High-Purity Results
- What safety features are included in the box type annealing atmosphere furnace? Ensure Operator and Equipment Protection
- What effects does a reducing atmosphere have on ceramic wares during firing? Unlock Deep Colors and Unique Finishes
- What are some specific use cases of retort furnaces? Essential for High-Temperature Atmospheric Control
- What are the controlled atmospheres for heat treatment? Master the Art of Material Transformation
- What are the key features of an atmosphere box furnace? Unlock Precise Heat Processing in Controlled Environments



















