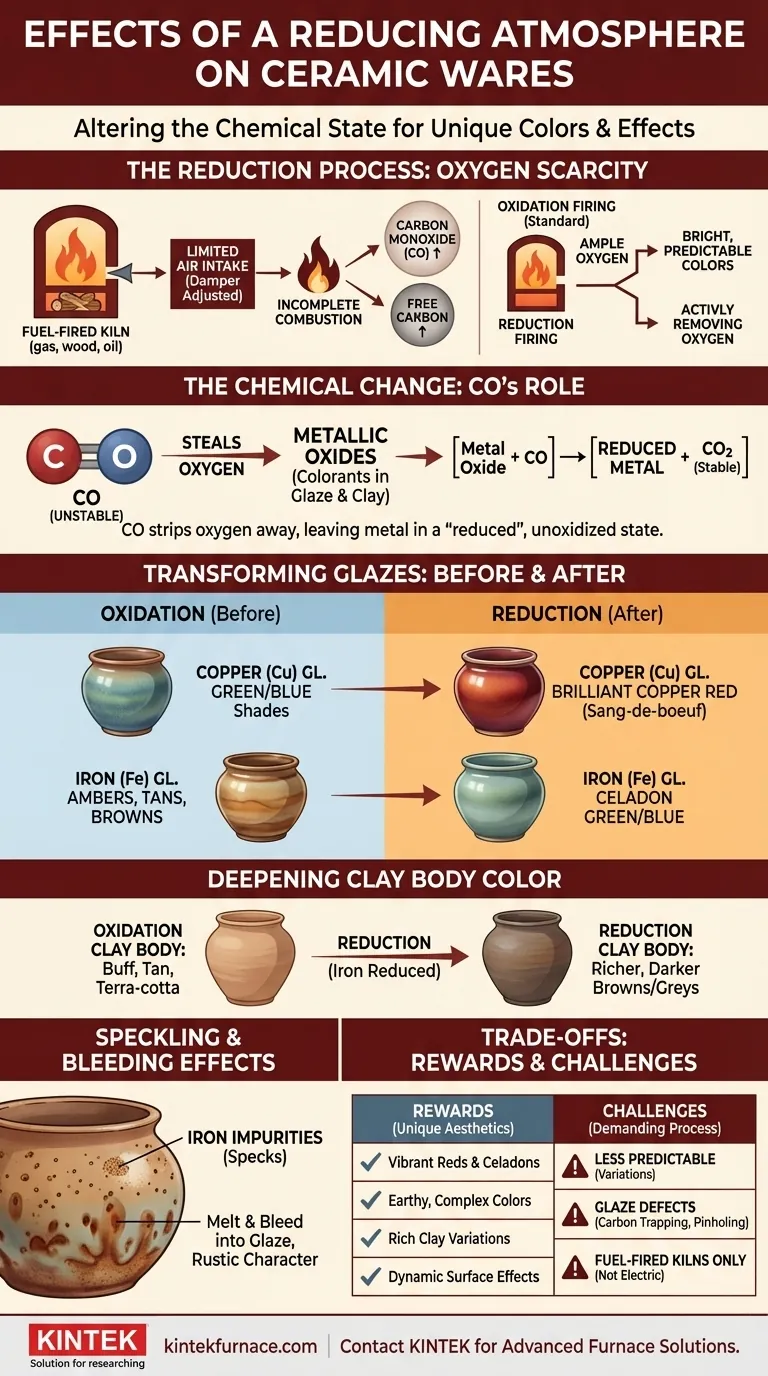
In essence, a reducing atmosphere fundamentally alters the chemical state of ceramic materials during firing. By intentionally starving the kiln of oxygen, you force a chemical reaction where carbon monoxide "steals" oxygen atoms from the metallic oxides present in both the clay body and the glazes, dramatically changing their final color and appearance.
While a standard (oxidation) firing adds oxygen to create bright, predictable colors, a reduction firing actively removes it. This process unlocks an entirely different palette of deep, complex, and often unpredictable colors by changing the fundamental chemistry of the metallic colorants.

The Chemistry of a Reducing Atmosphere
To control the effects of reduction, you must first understand the simple but powerful chemical process at play. It’s a deliberate manipulation of the kiln's environment to force a specific reaction.
Creating Oxygen Scarcity
In a fuel-fired kiln (gas, wood, or oil), a reducing atmosphere is created by limiting the air intake. This is typically done by adjusting a damper to reduce the draft.
This causes incomplete combustion of the fuel, which increases the level of free carbon and, more importantly, carbon monoxide (CO) within the kiln.
The Role of Carbon Monoxide
At high temperatures, carbon monoxide is chemically unstable and aggressively seeks an oxygen atom to become the more stable carbon dioxide (CO2).
It finds these oxygen atoms in the metallic oxides used as colorants in your glazes and clay. The CO effectively strips the oxygen away from the metal, leaving the metal in a "reduced," unoxidized state.
The Impact on Glazes and Clay Bodies
This chemical change is not subtle. It is responsible for some of the most sought-after and iconic effects in pottery.
Transforming Glaze Colors
The most dramatic effects of reduction are seen in glazes containing specific metal oxides.
- Copper (Cu): In an oxidizing atmosphere, copper oxide produces shades of green and blue. In reduction, that same copper is stripped of its oxygen and reverts to a metallic state, creating brilliant copper reds, known as sang-de-boeuf or "oxblood" glazes.
- Iron (Fe): Iron oxide is the workhorse colorant. In oxidation, it yields ambers, tans, and browns. In reduction, a small amount of iron can produce the delicate pale greens and blues of celadon glazes.
Deepening the Clay Body Color
Reduction doesn't just affect the glaze; it also transforms the clay itself. Most clay bodies contain a certain amount of iron.
In an oxidation firing, this iron makes the clay a buff, tan, or terra-cotta color. When fired in reduction, that same iron is reduced, causing the clay body to shift to a much richer, warmer, and often darker shade of brown or grey. This effect is known as body reduction.
Creating Speckling and Bleeding
For clay bodies with granular iron impurities (specks), reduction firing can cause these iron spots to melt and "bleed" up through the overlying glaze. This creates a speckled pattern that is often highly valued for its rustic and dynamic character.
Understanding the Trade-offs
While reduction can produce stunning results, it is a more demanding process than oxidation and comes with its own set of challenges.
The Challenge of Predictability
Reduction firing is inherently less uniform and predictable than oxidation. Small fluctuations in the kiln atmosphere can lead to significant variations in color, even on the same piece. Many artists embrace this variability as part of the aesthetic.
Risk of Glaze Defects
If reduction is initiated too early or is too intense, it can lead to problems. Carbon trapping can occur, where black carbon particles get sealed into the glaze, causing black spots, pinholing, or blistering.
Kiln and Fuel Constraints
True atmospheric reduction is only possible in fuel-fired kilns. Electric kilns operate with an inherently clean, oxygen-rich atmosphere. While some effects can be mimicked in an electric kiln using silicon carbide or placing combustibles in saggars, it is not the same as a true fuel-kiln reduction.
Making the Right Choice for Your Goal
The choice between an oxidation and reduction atmosphere depends entirely on your desired aesthetic outcome.
- If your primary focus is vibrant, predictable color: Oxidation firing is your most reliable path, providing stable and consistent results for a wide range of commercial glazes.
- If your primary focus is earthy, complex, and unique effects: Embrace reduction firing to achieve deep copper reds, subtle iron celadons, and rich variations that are impossible in oxidation.
- If your primary focus is rich, dark clay bodies: Employ body reduction to transform the iron within your clay, adding depth and warmth to your unglazed or lightly glazed surfaces.
Ultimately, choosing to fire in a reducing atmosphere is about trading absolute control for the possibility of profound and unique beauty.
Summary Table:
| Effect | Description |
|---|---|
| Glaze Color Change | Copper turns red, iron produces celadon greens/blues. |
| Clay Body Darkening | Iron in clay shifts to richer browns or greys. |
| Speckling and Bleeding | Iron impurities create rustic patterns in the glaze. |
| Predictability | Less uniform than oxidation, leading to unique variations. |
| Kiln Requirements | Only possible in fuel-fired kilns (gas, wood, oil). |
Ready to achieve stunning, custom ceramic finishes with precise high-temperature control? At KINTEK, we specialize in advanced furnace solutions like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong in-house R&D and manufacturing capabilities allow for deep customization to meet your unique experimental needs. Contact us today to discuss how our tailored high-temperature furnaces can elevate your ceramic firing processes and deliver exceptional results!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How does a chemically inert atmosphere function in a furnace? Prevent Oxidation and Ensure Material Purity
- How does the inert atmosphere heat treating process work? Prevent Oxidation for Superior Material Quality
- What is the use of nitrogen in furnace? Prevent Oxidation for Superior Heat Treatment
- How does an inert atmosphere prevent oxidation? Shield Materials from Oxygen Damage
- What are the benefits of inert atmosphere heat treating? Prevent Oxidation and Preserve Material Integrity



















