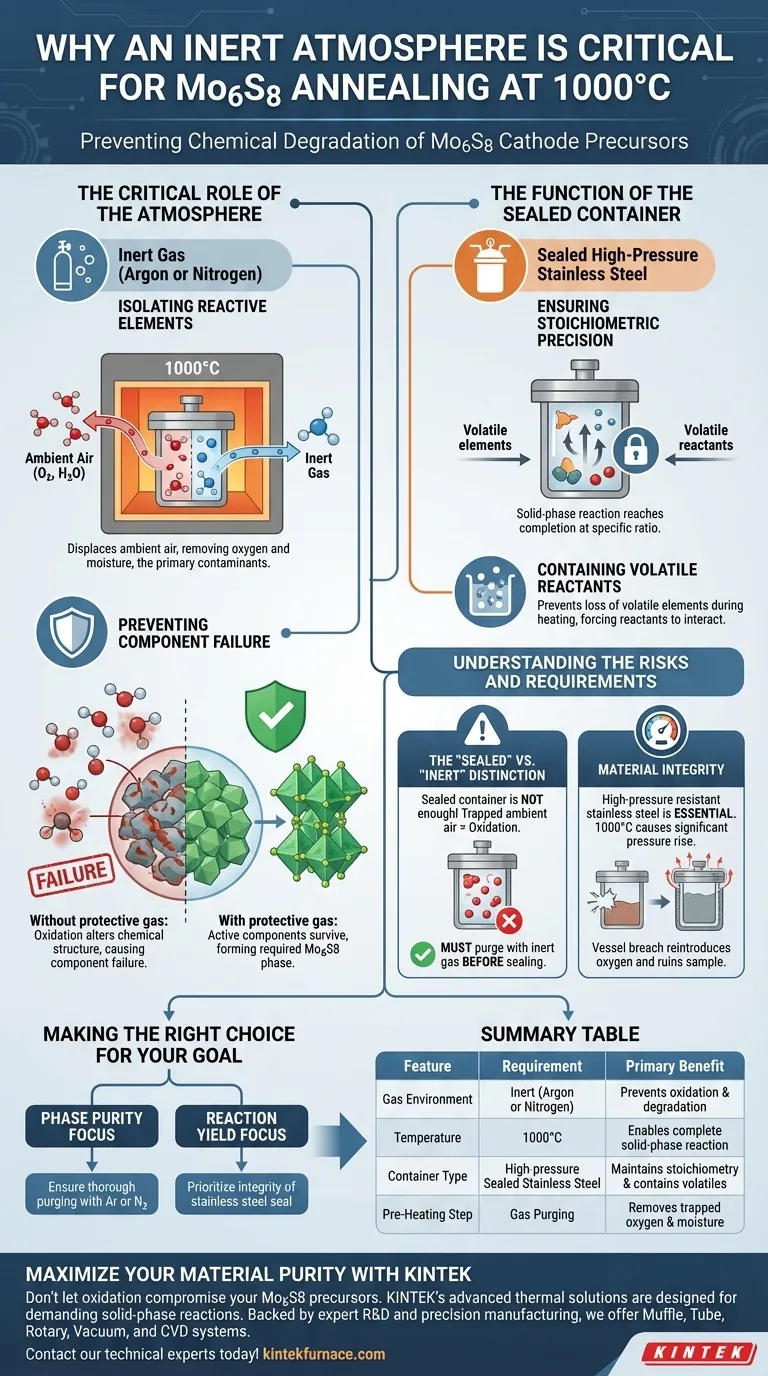
An inert atmosphere is strictly required to preventing the chemical degradation of Mo6S8 cathode precursors during the 1000°C annealing process. This specific environment neutralizes the threat of ambient air, isolating the materials from oxygen and moisture that would otherwise cause the active components to fail or oxidize rather than forming the desired compound.
The success of Mo6S8 synthesis relies on maintaining a precise chemical balance at high heat. The inert atmosphere acts as a protective shield, while the sealed container acts as a pressurized lock, ensuring reactants undergo a complete solid-phase reaction without interference from the outside environment.

The Critical Role of the Atmosphere
Isolating Reactive Elements
At 1000°C, the chemical reactivity of precursors increases significantly. An inert atmosphere, typically composed of argon or nitrogen, is introduced to displace ambient air. This effectively removes oxygen and moisture, which are the primary contaminants that ruin the synthesis process.
Preventing Component Failure
Without this protective gas layer, the active components in the precursor mix would oxidize immediately. Oxidation alters the chemical structure of the material, leading to the failure of the active components and preventing the formation of the specific Mo6S8 phase required for cathode performance.
The Function of the Sealed Container
Ensuring Stoichiometric Precision
The process utilizes a sealed, high-pressure resistant stainless steel container. The primary purpose of sealing the vessel is to ensure the solid-phase reaction reaches completion at a specific stoichiometric ratio.
Containing Volatile Reactants
By sealing the environment, you prevent the loss of volatile elements during the heating phase. This entrapment forces the reactants to interact with each other rather than escaping or reacting with the external atmosphere.
Understanding the Risks and Requirements
The "sealed" vs. "inert" distinction
It is a common misconception that a sealed container alone is sufficient. Even a perfectly sealed vessel will result in oxidation if it traps ambient air inside before sealing. You must purge the container with inert gas before sealing to ensure the internal environment is purely inert.
Material Integrity
The requirement for high-pressure resistant stainless steel is not arbitrary. The internal pressure rises significantly at 1000°C; using a vessel that cannot withstand this pressure will lead to a breach, reintroducing oxygen and ruining the sample.
Making the Right Choice for Your Goal
To ensure the successful synthesis of Mo6S8 cathode materials, you must align your equipment choices with the chemical requirements of the reaction.
- If your primary focus is Phase Purity: Ensure thorough purging with Argon or Nitrogen to completely remove trace moisture and oxygen before heating.
- If your primary focus is Reaction Yield: Prioritize the integrity of the stainless steel seal to maintain the exact stoichiometric ratio of reactants throughout the 1000°C cycle.
The combination of an inert gas purge and a pressure-resistant seal is the only way to guarantee the active components survive the thermal process intact.
Summary Table:
| Feature | Requirement for Mo6S8 Annealing | Primary Benefit |
|---|---|---|
| Gas Environment | Inert (Argon or Nitrogen) | Prevents oxidation and component degradation |
| Temperature | 1000°C | Enables complete solid-phase reaction |
| Container Type | High-pressure Sealed Stainless Steel | Maintains stoichiometry and contains volatile reactants |
| Pre-Heating Step | Gas Purging | Removes trapped oxygen and moisture prior to sealing |
Maximize Your Material Purity with KINTEK
Don't let oxidation compromise your Mo6S8 cathode precursors. KINTEK’s advanced thermal solutions are designed for demanding solid-phase reactions. Backed by expert R&D and precision manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable high-temperature lab furnaces tailored to your specific pressure and atmosphere requirements.
Ensure your research achieves its full potential with equipment built for reliability. Contact our technical experts today to discuss your custom furnace needs and secure the integrity of your high-temperature processes!
Visual Guide
References
- Andrijana Marojević, Jan Bitenc. Influence of Salt Concentration on the Electrochemical Performance of Magnesium Hexafluoroisopropoxy Aluminate Electrolyte. DOI: 10.1002/batt.202500497
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- Why is a strictly controlled argon (Ar) atmosphere required for Fe2O3@PDA pyrolysis? Ensure Superior Carbonization
- What type of pumps are used in low vacuum atmosphere furnaces? Rotary Vane Pumps for Efficient Heat Treatment
- What critical environmental conditions does a tube atmosphere furnace provide for t-BTO@C carbonization?
- What are some common applications of retort furnaces? Essential for Controlled Atmosphere Heat Treatment
- What type of vacuum pumps are used in low vacuum atmosphere furnaces? Reliable Rotary Vane Pumps for Cost-Effective Heating
- Can atmosphere furnaces be customized for specific applications? Unlock Precision for Your Unique Processes
- How does the experimental box type atmosphere furnace ensure accurate atmosphere control? Master Precise Gas Management for Reliable Results
- How are retort furnaces utilized in laboratory settings? Essential for Controlled Atmosphere Thermal Processes



















