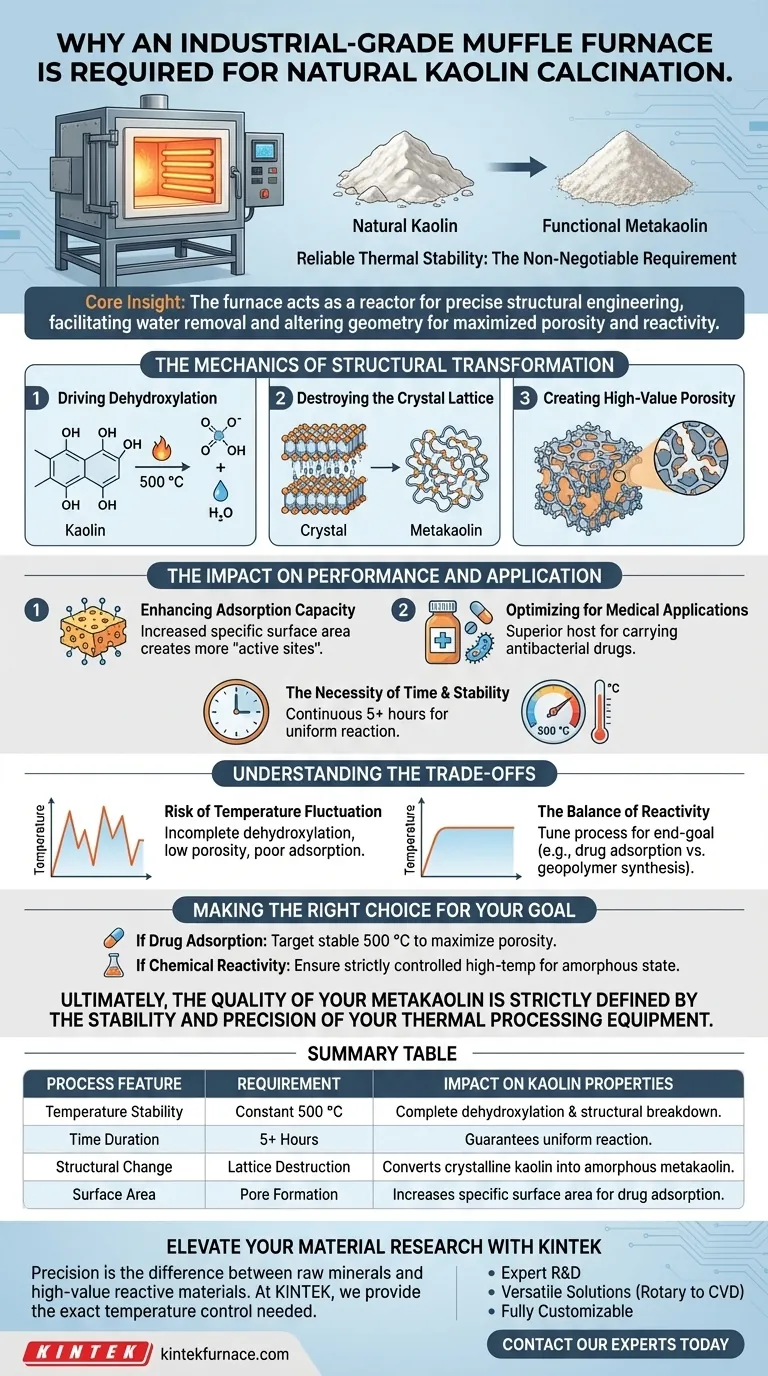
Reliable thermal stability is the non-negotiable requirement. To successfully treat natural kaolin, an industrial-grade muffle furnace is required to maintain a precise, stable high-temperature environment (specifically 500 °C) for extended durations, such as five hours. This apparatus ensures the consistent heat profile necessary to drive the chemical changes that convert raw kaolin into functional metakaolin.
The Core Insight The muffle furnace does more than simply heat the material; it acts as a reactor for precise structural engineering. By maintaining strict thermal control, the furnace facilitates the removal of internal water and hydroxyl groups, fundamentally altering the mineral's geometry to maximize porosity and reactivity.

The Mechanics of Structural Transformation
Driving the Dehydroxylation Reaction
The primary purpose of the furnace is to facilitate dehydroxylation. This is a chemical process where the high heat forces hydroxyl groups (-OH) and adsorbed water out of the kaolin structure.
destroying the Crystal Lattice
As the temperature holds, the rigid, layered crystal structure of natural kaolin begins to break down. This physical and chemical transformation converts the ordered mineral into metakaolin, an amorphous and highly reactive material.
Creating High-Value Porosity
The removal of water molecules leaves behind voids within the mineral's framework. This results in a significant increase in both porosity and specific surface area, which are the defining characteristics of high-quality metakaolin.
The Impact on Performance and Application
Enhancing Adsorption Capacity
The structural changes driven by the furnace directly correlate to the material's utility. The increased specific surface area creates more "active sites" on the mineral.
optimizing for Medical Applications
For specific applications, such as carrying antibacterial drugs, these active sites are critical. The calcined metakaolin acts as a superior host, offering enhanced adsorption capacity compared to raw kaolin.
The Necessity of Time and Stability
This transformation is not instantaneous. An industrial furnace is required to hold the temperature at exactly 500 °C for continuous periods (e.g., 5 hours) to ensure the reaction is uniform throughout the entire batch.
Understanding the Trade-offs
The Risk of Temperature Fluctuation
Precise control is paramount; treating kaolin is not merely about reaching a peak temperature, but maintaining it. If the temperature fluctuates or drops below the target (500 °C), the dehydroxylation will be incomplete, leaving the kaolin with low porosity and poor adsorption traits.
The Balance of Reactivity
While heat increases reactivity, the process must be tuned to the specific end-goal. For example, while 500 °C maximizes adsorption for drugs, other applications (like geopolymer synthesis) might utilize different thermal profiles to achieve specific amorphous states.
Making the Right Choice for Your Goal
When configuring your calcination process, your target temperature and duration should be dictated by the specific properties you need in the final material.
- If your primary focus is Drug Adsorption: Target a stable 500 °C cycle to maximize porosity and specific surface area for holding antibacterial agents.
- If your primary focus is Chemical Reactivity: Ensure the furnace is capable of strictly controlled high-temperature environments to fully destroy the crystal structure and achieve a highly amorphous state.
Ultimately, the quality of your metakaolin is strictly defined by the stability and precision of your thermal processing equipment.
Summary Table:
| Process Feature | Requirement | Impact on Kaolin Properties |
|---|---|---|
| Temperature Stability | Constant 500 °C | Ensures complete dehydroxylation & structural breakdown |
| Time Duration | 5+ Hours | Guarantees uniform reaction throughout the batch |
| Structural Change | Lattice Destruction | Converts crystalline kaolin into amorphous metakaolin |
| Surface Area | Pore Formation | Increases specific surface area for drug adsorption |
Elevate Your Material Research with KINTEK
Precision is the difference between raw minerals and high-value reactive materials. At KINTEK, we understand that your research depends on thermal stability. Our industrial-grade Muffle, Tube, and Vacuum furnaces provide the exact temperature control needed for sensitive processes like kaolin dehydroxylation.
Why choose KINTEK?
- Expert R&D: Systems designed for consistent, long-duration thermal cycles.
- Versatile Solutions: From Rotary to CVD systems, we cover all lab high-temp needs.
- Fully Customizable: Tailored configurations to meet your specific calcination and synthesis requirements.
Ready to achieve superior porosity and reactivity in your materials? Contact our experts today to find the perfect furnace for your laboratory.
Visual Guide
References
- Aruzhan Alimbek, Alyiya Ospanova. Synthesis and Antibacterial Evaluation of Chlorhexidine- and Triclosan-Impregnated Kaolinite Nanocomposites. DOI: 10.3390/ma18010174
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How does the temperature control system of a muffle furnace work? Achieve Precise Thermal Processing for Your Lab
- What safety measures should be observed around the muffle furnace? Essential Protocols for Safe Operation
- What key functions does a muffle furnace perform during the industrial analysis of coal samples? Optimize Proximate Analysis
- What were the results of annealing silicon-based materials in the muffle furnace? Boost Electrical Conductivity for Semiconductors
- How does a high-temperature box resistance furnace facilitate the heat treatment of FeAl alloys? Expert Solutions
- What specific PPE is recommended for loading and unloading a benchtop furnace? Essential Gear for Safe High-Temperature Handling
- Why is a muffle furnace required for alpha-Fe2O3? Unlock Precise Phase Transformation & High Crystallinity
- What is the core function of a high-temperature muffle furnace in silver nanoparticle circuits? Optimize Conductivity



















