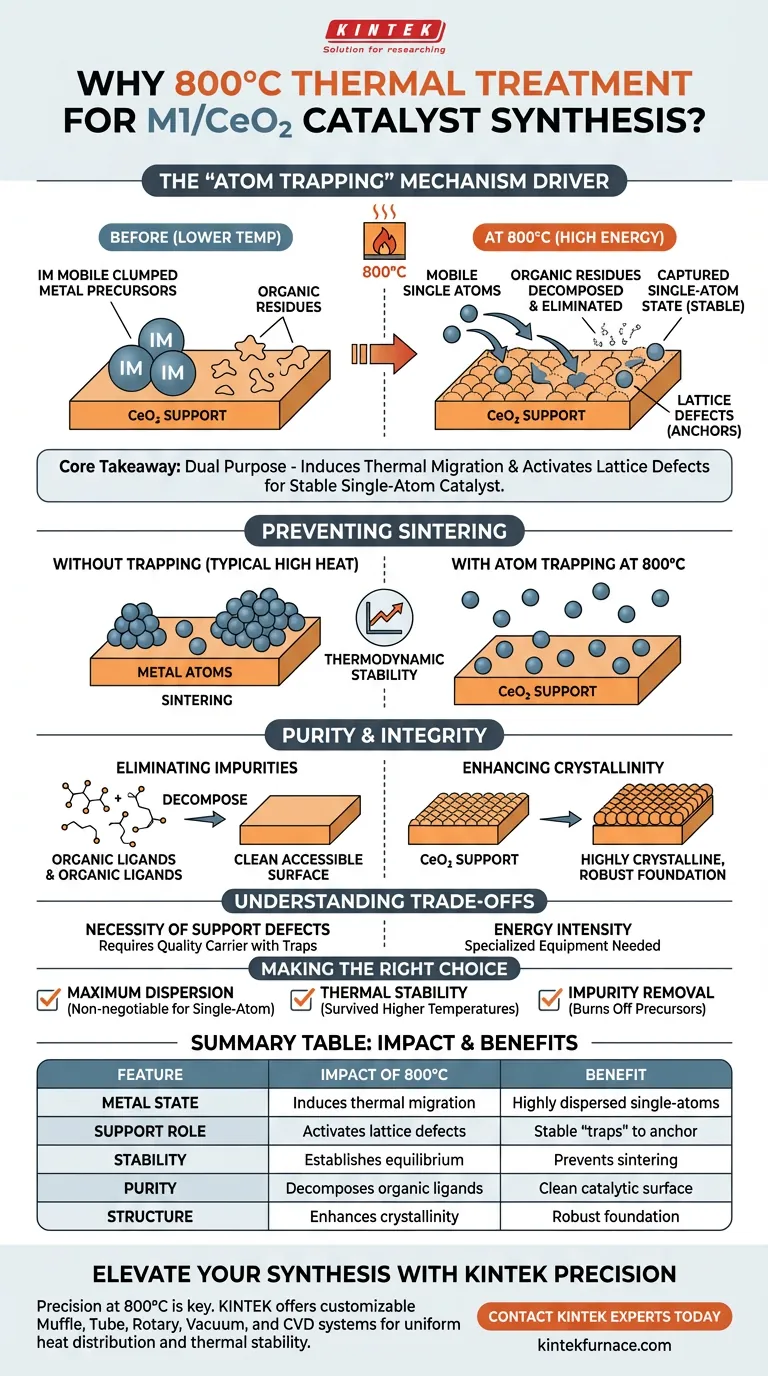
The 800°C thermal treatment is the primary driver of the "atom trapping" mechanism. This specific temperature provides the necessary energy to mobilize noble metal precursors across the surface of the cerium dioxide (CeO2) carrier. Once mobile, these metal atoms are captured by surface lattice defects, locking them into a stable, single-atom state rather than allowing them to aggregate into larger particles.
Core Takeaway The high-temperature environment serves a dual purpose: it induces the thermal migration of metal atoms while simultaneously activating the support’s lattice defects to trap them. This creates a thermodynamically stable, highly dispersed single-atom catalyst that resists the sintering typically caused by extreme heat.
The Mechanism of Atom Trapping
Inducing Thermal Migration
At lower temperatures, metal precursor atoms often remain static or clumped where they were deposited. The 800°C thermal field provides the kinetic energy required to break these initial bonds.
This energy forces the metal precursors to migrate across the surface of the carrier. This mobility is a prerequisite for the atoms to locate the specific sites where they will be most effective.
Utilizing Lattice Defects as Anchors
The cerium dioxide (CeO2) carrier is not a perfect crystal; it contains specific surface lattice defects. As the metal atoms migrate, they encounter these defects.
These defects act as "traps" or anchors. Because the interaction between the metal atom and the defect is energetically favorable, the atom is captured and stabilized instantly upon contact.
Preventing Metal Sintering
Without this specific trapping mechanism, high temperatures usually cause metal atoms to merge and form large clusters, a process known as sintering. Sintering drastically reduces the catalytic surface area.
By utilizing the atom trapping method at 800°C, the metal remains dispersed as isolated single atoms. This defies the natural tendency of metals to aggregate under heat.
Purity and Structural Integrity
Eliminating Residual Impurities
The synthesis process often uses ligands, such as citric acid, to initially coordinate the metals. These organic residues can block active sites if left behind.
The high-temperature treatment completely decomposes these organic ligands and impurities. This ensures the final catalyst surface is clean and fully accessible for reactions.
Enhancing Crystallinity and Stability
Exposure to 800°C ensures that the CeO2 support transforms into a highly crystalline state. This structural rigidity provides a robust foundation for the metal atoms.
Furthermore, because the catalyst is synthesized at such a high temperature, it possesses inherent thermodynamic stability. It is less likely to degrade when used in practical applications that operate at elevated temperatures.
Understanding the Trade-offs
The Necessity of Support Defects
This method relies entirely on the quality of the carrier. If the CeO2 support lacks sufficient lattice defects, the high temperature will fail to trap the atoms.
Without enough "traps," the 800°C heat will backfire, causing the mobile metal atoms to collide and sinter into large, inactive particles.
Energy Intensity
Maintaining a furnace at 800°C is energy-intensive. It requires specialized equipment capable of maintaining a stable, uniform thermal field to ensure consistent results across the entire batch.
Making the Right Choice for Your Goal
This synthesis method is designed for high-performance applications where stability and dispersion are paramount.
- If your primary focus is Maximum Dispersion: The 800°C treatment is non-negotiable, as it powers the migration required to reach single-atom distribution.
- If your primary focus is Thermal Stability: Use this method to ensure the catalyst has already survived temperatures higher than its likely operating environment.
- If your primary focus is Impurity Removal: This treatment effectively burns off all organic precursors that could inhibit catalytic activity.
The 800°C treatment transforms thermal energy from a destructive force into a constructive tool for atomic precision.
Summary Table:
| Feature | Impact of 800°C Thermal Treatment | Benefit for M1/CeO2 Synthesis |
|---|---|---|
| Metal State | Induces thermal migration to lattice defects | Creates highly dispersed single-atom catalysts |
| Support Role | Activates CeO2 surface lattice defects | Provides stable 'traps' to anchor metal atoms |
| Stability | Establishes thermodynamic equilibrium | Prevents metal sintering and catalyst degradation |
| Purity | Decomposes organic ligands/impurities | Ensures a clean, fully accessible catalytic surface |
| Structure | Enhances CeO2 crystallinity | Provides a robust and rigid structural foundation |
Elevate Your Catalyst Synthesis with KINTEK Precision
Precision at 800°C is the difference between an aggregated cluster and a high-performance single-atom catalyst. At KINTEK, we understand that thermal stability and uniform heat distribution are non-negotiable for advanced materials research.
Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. Our lab high-temperature furnaces are fully customizable to meet your unique synthesis requirements, ensuring you achieve the exact 'atom trapping' environment your research demands.
Ready to optimize your thermal treatment process?
Contact KINTEK Experts Today to find the perfect furnace solution for your laboratory.
Visual Guide
References
- Jinshu Tian, Yong Wang. NO Reduction with CO on Low‐loaded Platinum‐group Metals (Rh, Ru, Pd, Pt, and Ir) Atomically Dispersed on Ceria. DOI: 10.1002/cctc.202301227
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How do quartz tubes contribute to energy efficiency? Boost Your Lab's Thermal Performance
- What are the main differences in performance between MoSi2 and SiC heating elements? Choose the Right Element for Your High-Temp Needs
- Why is ductility a necessary property for heating elements? Ensure Reliability and Manufacturing Success
- What happens when a ceramic heating element reaches its preset temperature? Discover Self-Regulating Safety and Efficiency
- What are the typical shapes of MoSi2 heating elements? Explore U, W, L Shapes for Optimal Furnace Performance
- What are the typical applications of stainless steel sheaths in heating elements? Optimize Performance and Durability
- What are the key differences between SiC and MoSi2 heating elements in sintering furnaces? Choose the Right Element for Your High-Temp Needs
- What are the primary applications of MoSi2 heating elements in research? Achieve Reliable High-Temp Control for Material Synthesis



















