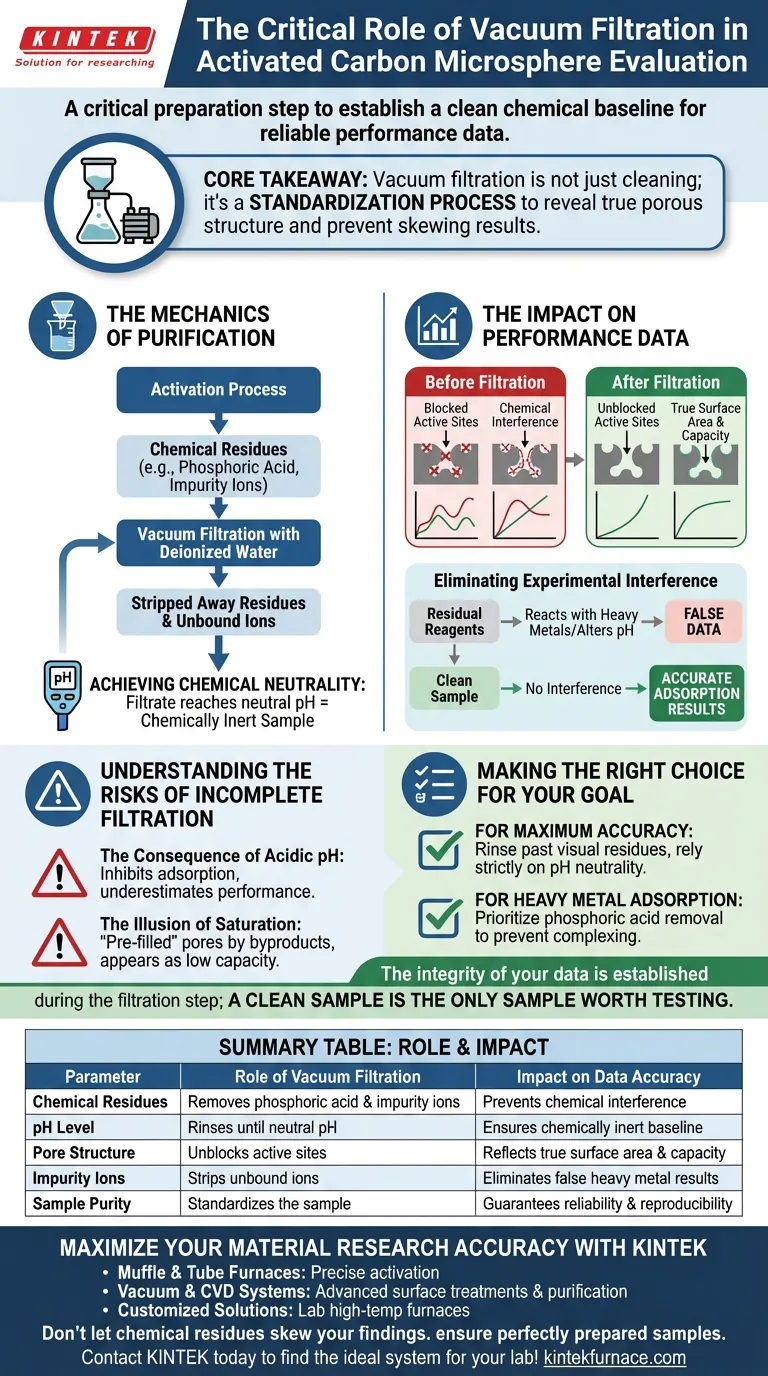
A vacuum filtration system is a critical preparation step required to establish a clean chemical baseline before testing begins. It facilitates the thorough rinsing of activation products with deionized water, specifically targeting the removal of residual phosphoric acid and impurity ions. This process is continued until the filtrate reaches a neutral pH, ensuring the material is chemically inert and ready for accurate evaluation.
Core Takeaway The reliability of activated carbon performance data depends entirely on sample purity. Vacuum filtration is not merely a cleaning step; it is a standardization process that exposes the material’s true porous structure and prevents leftover activation chemicals from skewing subsequent adsorption test results.

The Mechanics of Purification
Removal of Chemical Residues
During the activation process, chemical agents like phosphoric acid are introduced to develop porosity.
Once activation is complete, these agents become contaminants. Vacuum filtration drives deionized water through the sample, effectively stripping away residual phosphoric acid and unbound impurity ions that would otherwise cling to the carbon structure.
Achieving Chemical Neutrality
The filtration process must continue until the filtrate indicates a neutral pH.
This is the definitive metric for cleanliness. A neutral pH confirms that the acidic activation agents have been successfully flushed out, preventing them from altering the local chemical environment during later experiments.
The Impact on Performance Data
Unblocking Active Sites
The performance of activated carbon is defined by its available surface area.
Residual chemicals can physically block or chemically occupy the "active sites" on the surface of the carbon microspheres. Filtration exposes these sites, ensuring that the surface area measured reflects the carbon's actual capacity, not a chemically clogged artifact.
Eliminating Experimental Interference
The user’s ultimate goal is often to test the carbon's ability to adsorb heavy metal ions.
If residual reagents remain, they can react with heavy metals or alter the pH of the test solution. This creates chemical interference, leading to false data regarding the carbon's adsorption efficiency. Vacuum filtration eliminates this variable, ensuring that observed results are due to the carbon's pore structure alone.
Understanding the Risks of Incomplete Filtration
The Consequence of acidic pH
If the vacuum filtration process is terminated too early, the sample remains acidic.
In adsorption experiments, pH is a controlling factor. An acidic sample will artificially influence the behavior of heavy metal ions, likely inhibiting adsorption and resulting in data that underestimates the material's true performance.
The Illusion of Saturation
Failure to remove impurities leads to a "pre-filled" pore structure.
When you test the material, it will appear to saturate quickly. This is not because the material has low capacity, but because a significant portion of its capacity is already occupied by leftover activation byproducts.
Making the Right Choice for Your Goal
If your primary focus is Maximum Accuracy:
- Ensure rinsing continues well past the visual disappearance of residues; rely strictly on pH measurements of the filtrate to confirm neutrality.
If your primary focus is Heavy Metal Adsorption:
- Prioritize the removal of phosphoric acid traces, as these specific ions can chemically complex with metals and invalidate your capture rate data.
The integrity of your data is established during the filtration step; a clean sample is the only sample worth testing.
Summary Table:
| Parameter | Role of Vacuum Filtration | Impact on Data Accuracy |
|---|---|---|
| Chemical Residues | Removes phosphoric acid & impurity ions | Prevents chemical interference in adsorption tests |
| pH Level | Rinses until filtrate reaches neutral pH | Ensures material is chemically inert for baseline testing |
| Pore Structure | Unblocks active sites on the carbon surface | Reflects true surface area & capacity measurements |
| Impurity Ions | Strips unbound ions from the structure | Eliminates false results in heavy metal adsorption |
| Sample Purity | Standardizes the sample for evaluation | Guarantees reliability and reproducibility of results |
Maximize Your Material Research Accuracy with KINTEK
Precise material evaluation starts with high-quality preparation and thermal processing. KINTEK provides the industry-leading laboratory equipment necessary to transform raw materials into high-performance catalysts and adsorbents.
Backed by expert R&D and manufacturing, we offer:
- Muffle & Tube Furnaces: For precise activation and carbonization.
- Vacuum & CVD Systems: For advanced surface treatments and purification.
- Customized Solutions: Lab high-temp furnaces tailored to your unique research needs.
Don't let chemical residues skew your findings. Ensure your samples are perfectly prepared for testing. Contact KINTEK today to find the ideal system for your lab!
Visual Guide
References
- Saeed Alhawtali, Chun‐Yang Yin. Date Palm Leaflet-Derived Carbon Microspheres Activated Using Phosphoric Acid for Efficient Lead (II) Adsorption. DOI: 10.3390/c10010026
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Stainless Steel Quick Release Vacuum Chain Three Section Clamp
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What is the technical significance of using a quartz boat in 2D superlattice preparation? Enhancing CVD Precision
- Why is the integration of a K-type thermocouple and a data logger necessary for Vanadis 60 steel? Unlock Precision.
- Why is it necessary to achieve a vacuum level of 3 x 10^-2 mm Hg for quartz tube sealing? Ensure Safety and Purity
- Why is a tantalum (Ta) crucible essential for Li3-3xScxSb sintering? Ensure Pure Phase Stability at 1143 K
- What role do refractory bricks and graphite paper play within a quartz tube? Optimize RuMoOx/NC Synthesis Efficiency
- Why are sealed Niobium (Nb) tubes utilized as reaction vessels during the high-temperature solid-state synthesis of Ba1-xEuxZn2Sb2?
- What is the function of a high-purity Argon (Ar) gas flow control system? Ensure Superior Nanowire Uniformity
- Why is it necessary to use a mechanical vacuum pump for SnSe growth? Ensure High-Purity Material Synthesis



















