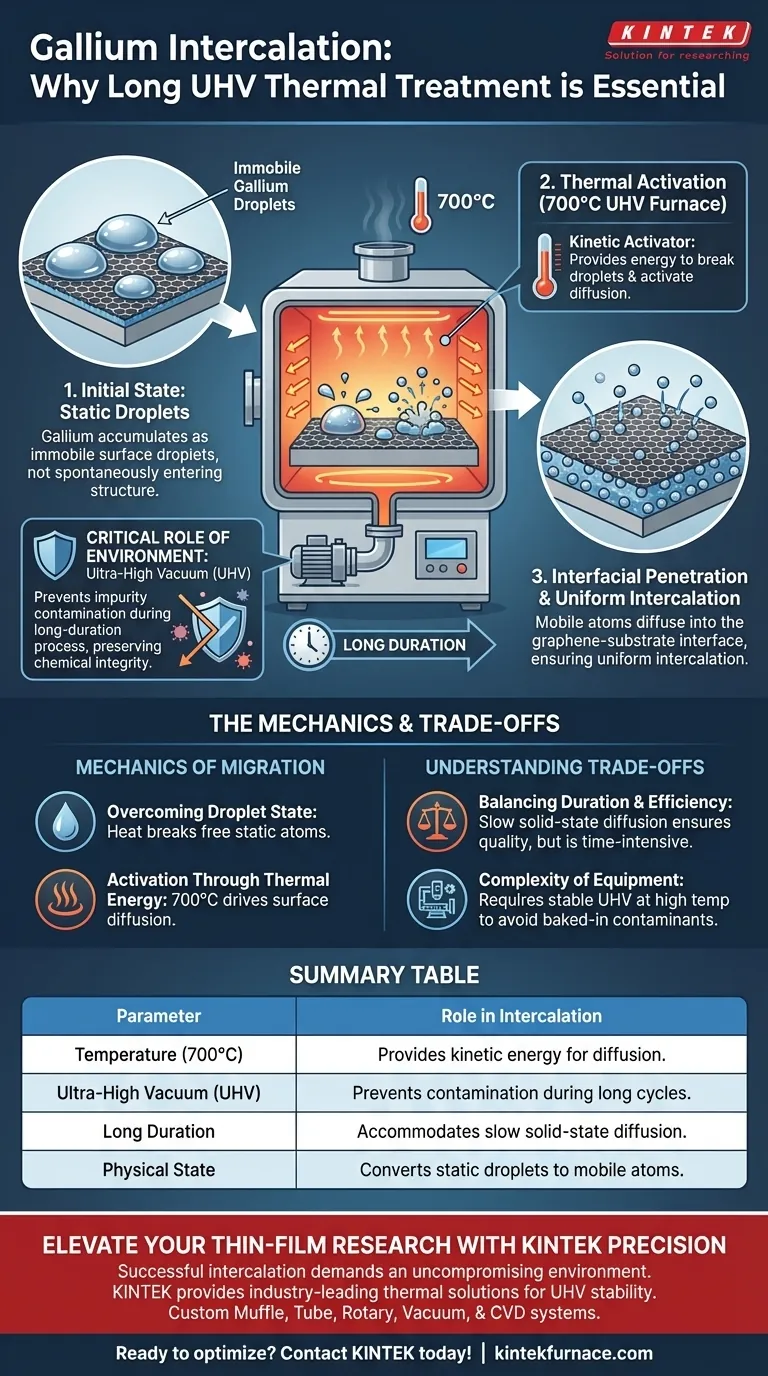
A long-duration thermal treatment is essential because gallium atoms initially accumulate as immobile droplets on the surface rather than spontaneously entering the material structure. The annealing furnace supplies the necessary thermal energy to activate these atoms, driving them from the surface into the interface between the graphene and the substrate, while the vacuum environment maintains purity.
The treatment acts as a kinetic activator, converting static surface droplets into mobile atoms that diffuse into the graphene interface. This extended process relies on Ultra-High Vacuum (UHV) to prevent contamination while the high heat drives uniform intercalation.

The Mechanics of Gallium Migration
Overcoming the Droplet State
Immediately following deposition, gallium does not automatically form the desired intercalated layer.
Instead, the atoms exist primarily as droplets resting on the surface of the material. Without intervention, these droplets would remain on top rather than penetrating the structure.
Activation Through Thermal Energy
The UHV annealing furnace provides a controlled high-temperature environment, ramping up to 700°C.
This specific level of thermal energy is required to activate surface diffusion. It provides the kinetic force necessary to break the atoms free from the surface droplets.
Interfacial Penetration
Once mobilized by the heat, the gallium atoms begin to migrate.
The thermal treatment drives these atoms to penetrate the interface between the graphene and the substrate. This migration is what ultimately ensures the uniform intercalation of the material.
The Critical Role of Environment
Why Ultra-High Vacuum (UHV) Matters
The diffusion of gallium from droplets into the interface is not an instantaneous event; it is a long-duration process.
Because the sample is exposed to high heat for an extended period, it is highly susceptible to reaction with the atmosphere.
The Ultra-High Vacuum is strictly necessary to prevent impurity contamination. It preserves the chemical integrity of the graphene and gallium during the slow diffusion process.
Understanding the Trade-offs
Balancing Duration and Efficiency
While this method ensures high-quality intercalation, the requirement for a "long duration" introduces efficiency constraints.
The process is time-intensive because it relies on solid-state diffusion, which is inherently slower than direct deposition methods.
Complexity of Equipment
Maintaining a UHV environment at 700°C for extended periods places high demands on equipment.
You must ensure your furnace maintains a stable vacuum at these temperatures, as even minor fluctuations can introduce contaminants that the heat will bake into the interface.
Making the Right Choice for Your Goal
To ensure successful gallium intercalation, you must align your processing parameters with the physical requirements of the atoms.
- If your primary focus is Uniformity: Ensure the thermal treatment reaches and maintains 700°C to fully activate diffusion and eliminate surface droplets.
- If your primary focus is Sample Purity: rigorous UHV maintenance is non-negotiable, as the extended duration increases the window of opportunity for contamination.
Mastering this process requires viewing heat as the engine of migration and vacuum as the shield of quality.
Summary Table:
| Parameter | Role in Intercalation |
|---|---|
| Temperature (700°C) | Provides kinetic energy to break surface droplets and activate diffusion. |
| Ultra-High Vacuum (UHV) | Prevents chemical contamination and atmospheric reaction during long cycles. |
| Long Duration | Accommodates the slow pace of solid-state diffusion into the graphene interface. |
| Physical State | Converts static surface gallium droplets into mobile, intercalating atoms. |
Elevate Your Thin-Film Research with KINTEK Precision
Successful gallium intercalation demands an uncompromising environment. KINTEK provides industry-leading thermal solutions designed to maintain ultra-high vacuum stability even during extended high-temperature cycles.
Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable for your unique lab requirements. Whether you are perfecting graphene interfaces or advanced semiconductor doping, our furnaces deliver the uniformity and purity your research depends on.
Ready to optimize your intercalation process? Contact KINTEK today to consult with our specialists!
Visual Guide
References
- Emanuele Pompei, Stefano Veronesi. Novel Structures of Gallenene Intercalated in Epitaxial Graphene. DOI: 10.1002/smll.202505640
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- How do process speeds compare between low vacuum and high vacuum furnaces? Uncover the Speed vs. Purity Trade-Off
- What is the significance of using a vacuum drying oven for silicon electrode slurries? Achieve Robust Battery Integrity
- What are the common types of materials used for constructing heating elements in electrically heated vacuum furnaces? Choose the Right Material for Your Process
- What are the advantages of vacuum heat treatment? Achieve Superior Material Quality and Control
- What is the specific purpose of using a vacuum high-temperature furnace for powder pre-treatment? Ensure Sharp Interfaces
- What processes can horizontal vacuum furnaces be used for? Unlock Versatile Thermal Applications
- What are the advantages of vacuum heat treatment? Achieve Superior Metallurgical Control and Pristine Surfaces
- Why is vacuum heat treatment essential for mirror copper tubes? Ensure Strength and Purity for High-Performance Applications



















