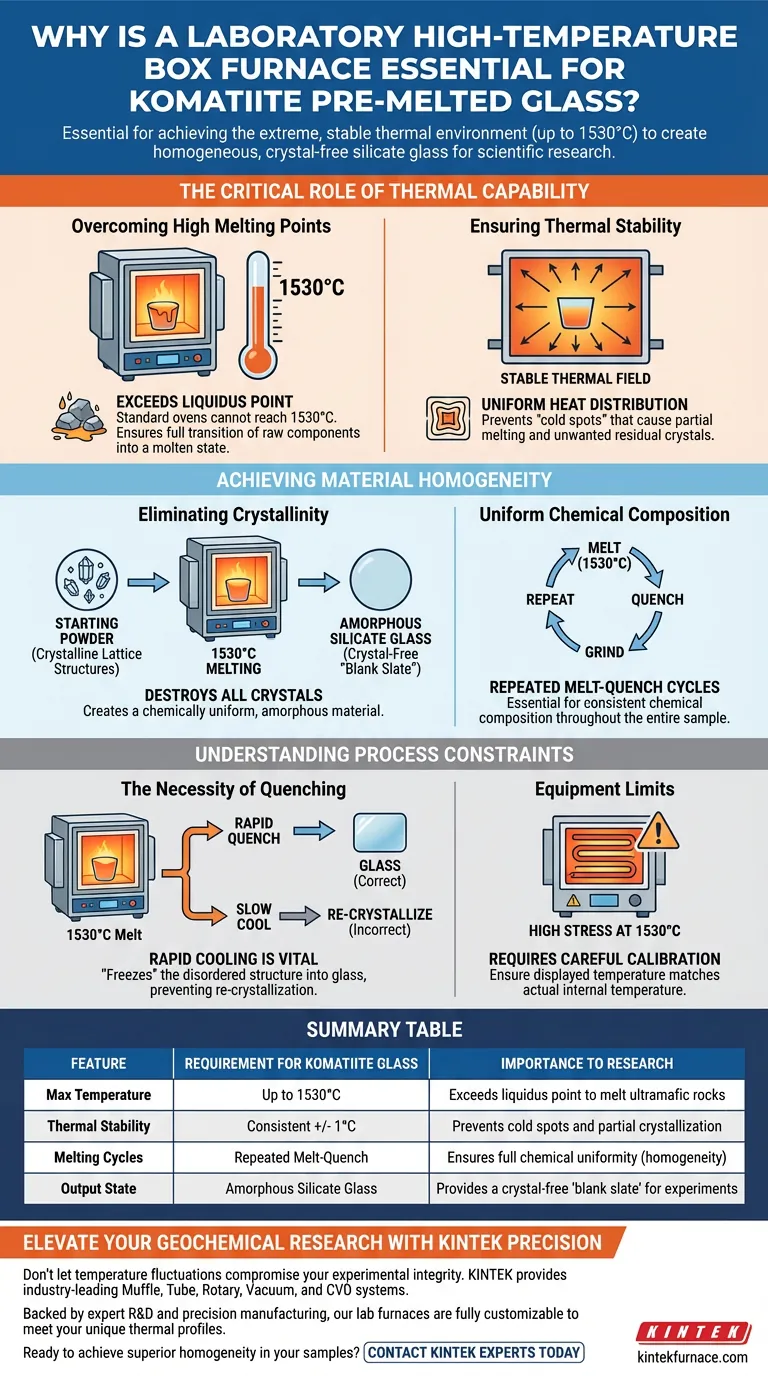
A laboratory high-temperature box furnace is essential for preparing komatiite pre-melted glass because it provides the extreme, stable thermal environment—specifically up to 1530°C—required to fully melt the rock's components. Without this precise high-temperature capability, it is impossible to transform the raw starting materials into the homogeneous, crystal-free silicate glass necessary for valid scientific experimentation.
The furnace facilitates the rigorous melting and quenching cycles required to eliminate all crystalline structures. This creates a chemically uniform "blank slate" material, which serves as the non-negotiable foundation for accurate phase equilibrium experiments.

The Critical Role of Thermal Capability
Overcoming High Melting Points
To successfully prepare komatiite glass, you must exceed the liquidus temperature of the starting materials. A standard laboratory oven cannot reach the extreme threshold required for these ultramafic rocks. The high-temperature box furnace is specifically engineered to achieve 1530°C, ensuring the raw components are fully transitioned into a molten state.
Ensuring Thermal Stability
Reaching the target temperature is only the first step; maintaining it is equally critical. The box furnace provides a stable thermal field, ensuring that the heat is distributed evenly around the crucible. This prevents cold spots that could result in partial melting, which would leave unwanted residual crystals in the mixture.
Achieving Material Homogeneity
Eliminating Crystallinity
The primary scientific goal in this process is to produce silicate glass, which is amorphous by definition. The high heat of the furnace ensures that all crystalline lattice structures in the starting powder are completely destroyed. If the furnace fails to fully melt the sample, remaining crystals will act as nucleation sites, compromising the validity of future experiments.
Uniform Chemical Composition
Preparing pre-melted glass is rarely a "one-and-done" process. To ensure the chemical composition is consistent throughout the entire sample, the material must undergo repeated high-temperature melting and subsequent quenching. The furnace enables researchers to melt the sample, remove it for quenching and grinding, and then return it to 1530°C multiple times until perfect uniformity is achieved.
Understanding the Process Constraints
The Necessity of Quenching
While the furnace is responsible for the melting phase, it works in tandem with the quenching process. The furnace creates the melt, but the operator must rapidly cool that melt to "freeze" the disordered atomic structure into glass. If the transition from the 1530°C environment to room temperature is too slow, the material will re-crystallize, negating the work done by the furnace.
Equipment Limits
Operating at 1530°C places significant stress on heating elements and insulation. While the furnace is essential, it requires careful calibration to ensure the displayed temperature matches the actual internal temperature. A discrepancy of even a few degrees at this extreme range can affect the viscosity and homogeneity of the melt.
Making the Right Choice for Your Goal
To ensure your komatiite preparation leads to successful equilibrium experiments, consider the following:
- If your primary focus is material purity: Prioritize a furnace with excellent temperature uniformity to prevent localized crystallization during the melt phase.
- If your primary focus is experimental reproducibility: Establish a strict protocol for the number of melt-quench cycles used, ensuring the furnace returns to the exact 1530°C setpoint every time.
By providing a reliable 1530°C thermal field, the high-temperature box furnace ensures your starting material is chemically uniform and structurally amorphous, safeguarding the integrity of your research.
Summary Table:
| Feature | Requirement for Komatiite Glass | Importance to Research |
|---|---|---|
| Max Temperature | Up to 1530°C | Exceeds liquidus point to melt ultramafic rocks |
| Thermal Stability | Consistent +/- 1°C | Prevents cold spots and partial crystallization |
| Melting Cycles | Repeated Melt-Quench | Ensures full chemical uniformity (homogeneity) |
| Output State | Amorphous Silicate Glass | Provides a crystal-free 'blank slate' for experiments |
Elevate Your Geochemical Research with KINTEK Precision
Don't let temperature fluctuations compromise your experimental integrity. KINTEK provides industry-leading Muffle, Tube, Rotary, Vacuum, and CVD systems specifically engineered to handle the rigorous demands of high-temperature material synthesis.
Backed by expert R&D and precision manufacturing, our lab furnaces are fully customizable to meet your unique thermal profiles—ensuring your komatiite melts reach the perfect 1530°C threshold every time.
Ready to achieve superior homogeneity in your samples?
Visual Guide
References
- Erin Keltie, James M. Brenan. Experiments and Models Bearing on the Role of Magma Mixing and Contamination on Chromite Crystallization in Ultramafic Magmas. DOI: 10.1093/petrology/egaf076
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What function does a muffle furnace perform during the air calcination of ZnO-Co3O4? Optimize Your Nanocomposites
- How is a high-temperature box furnace utilized during the calcination and sintering stages of SrVO3 precursors?
- How does a high-precision furnace enhance EIS testing for niobium-doped titanium dioxide? Achieve Accurate Material Data
- How does the price of a muffle furnace vary? Find the Perfect Fit for Your Lab's Budget
- What PPE is necessary when adjusting controls or handling equipment during furnace operation? Essential Safety Gear for High-Temperature Tasks
- Why is temperature uniformity important in a muffle furnace? Ensure Precise and Reliable Results
- How does a muffle furnace function and what is its primary purpose? Discover Precision Heating for Pure Results
- Why are muffle furnaces particularly suitable for ashing processes? Achieve Contaminant-Free Sample Analysis



















