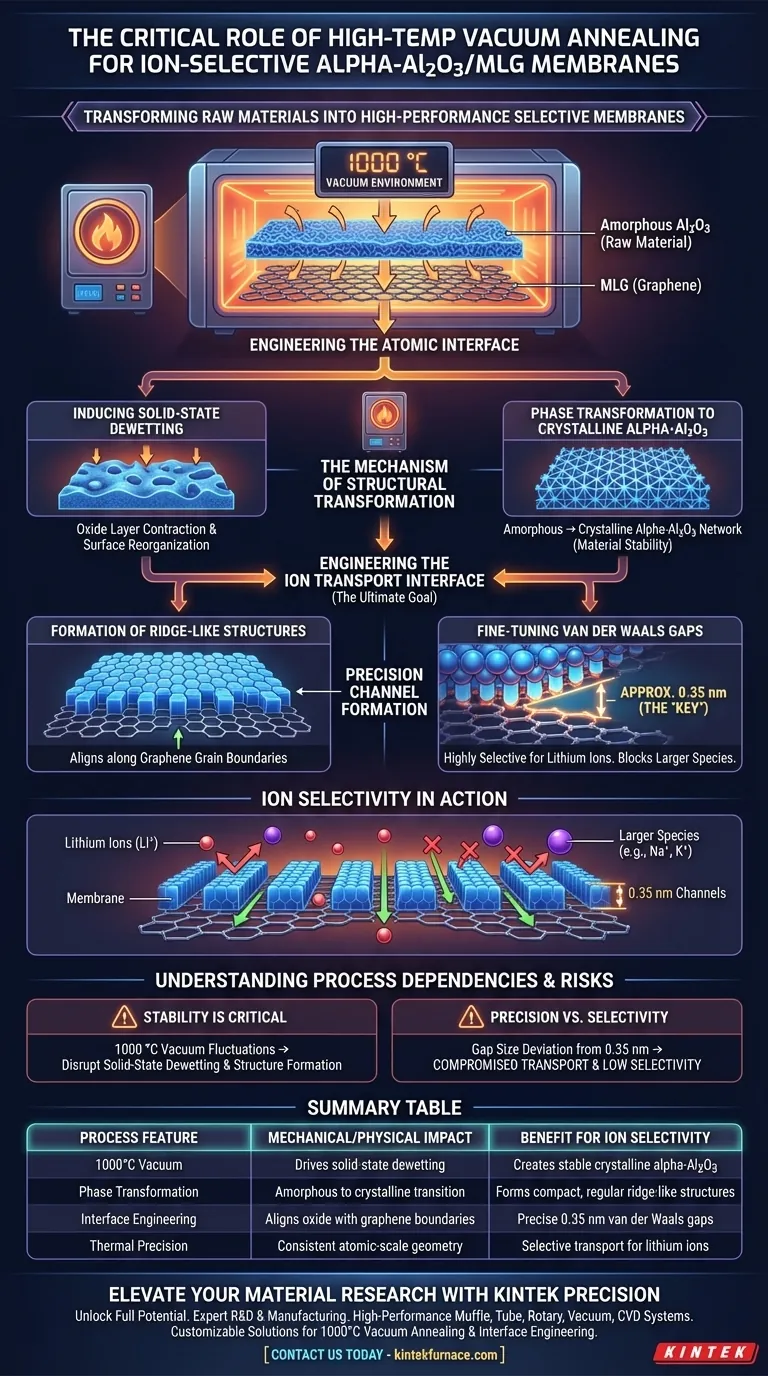
The high-temperature vacuum annealing furnace is the critical enabler for transforming raw materials into high-performance ion-selective membranes. It provides a stable 1000 °C vacuum environment that forces amorphous aluminum oxide (Al2O3) to undergo phase transformation and contraction. This specific thermal process creates precise physical channels that differentiate between ions based on size.
By driving solid-state dewetting and crystallization, the furnace engineers the material interface at the atomic level. This process fine-tunes the van der Waals gaps to approximately 0.35 nm, creating a physical "sieve" that is highly selective for lithium ions.

The Mechanism of Structural Transformation
To understand why this specific equipment is necessary, we must look at the physical changes occurring within the material at 1000 °C.
Inducing Solid-State Dewetting
The furnace creates a strictly controlled environment that triggers solid-state dewetting.
During this phase, the heat treatment forces the oxide layer to contract. This contraction is not a defect; it is a necessary step to reorganize the material's surface morphology.
Phase Transformation to Crystalline Alpha-Al2O3
Initially, the aluminum oxide exists in an amorphous (disordered) state.
The stable high-temperature environment facilitates a phase transformation, converting the amorphous material into a structured, crystalline alpha-Al2O3 network. Without the sustained heat and vacuum of the furnace, this crystallization—and the resulting material stability—would not occur.
Engineering the Ion Transport Interface
The ultimate goal of using this furnace is to construct transport channels with extreme precision. The heat treatment dictates the geometry of these channels.
Formation of Ridge-Like Structures
As the oxide crystallizes, it forms compact, regular ridge-like structures.
These structures do not form randomly; they align specifically along the graphene grain boundaries (MLG). This alignment is critical for creating a consistent interface between the two materials.
Fine-Tuning Van der Waals Gaps
The most critical outcome of this thermal process is the manipulation of the heterojunction interface.
The furnace allows for the precise fine-tuning of the van der Waals gaps between the oxide and the graphene. The process targets a specific gap size of approximately 0.35 nm. This dimension is the "key" that unlocks high selectivity, allowing lithium ions to pass while blocking larger species.
Understanding the Process Dependencies
While the furnace enables high performance, it also introduces strict dependencies regarding process control.
The Necessity of Environmental Stability
The formation of the alpha-Al2O3 network relies on the stability of the 1000 °C vacuum.
Any fluctuation in temperature or pressure could disrupt the solid-state dewetting process. Inconsistent heat treatment would fail to produce the compact, regular ridge-like structures required for the target gap size.
Precision vs. Selectivity
The selectivity of the membrane is directly tied to the precision of the annealing process.
If the van der Waals gaps deviate significantly from the 0.35 nm target, the membrane's ability to act as a selective transport channel is compromised. The furnace is not just heating the material; it is manufacturing a precise atomic-scale geometry.
Making the Right Choice for Your Goal
When evaluating the role of thermal processing in membrane fabrication, consider your specific material objectives.
- If your primary focus is structural integrity: Ensure your process can maintain a stable 1000 °C environment to drive the complete phase transformation from amorphous to crystalline alpha-Al2O3.
- If your primary focus is ion selectivity: Prioritize process controls that guarantee the formation of uniform ridge-like structures to achieve the critical 0.35 nm van der Waals gap.
The high-temperature vacuum annealing furnace is the precise tool required to bridge the gap between raw amorphous materials and highly selective, crystalline ion transport networks.
Summary Table:
| Process Feature | Mechanical/Physical Impact | Benefit for Ion Selectivity |
|---|---|---|
| 1000°C Vacuum | Drives solid-state dewetting | Creates stable crystalline alpha-Al2O3 |
| Phase Transformation | Amorphous to crystalline transition | Forms compact, regular ridge-like structures |
| Interface Engineering | Aligns oxide with graphene boundaries | Precise 0.35 nm van der Waals gaps |
| Thermal Precision | Consistent atomic-scale geometry | Selective transport for lithium ions |
Elevate Your Material Research with KINTEK Precision
Unlock the full potential of your ion-selective membranes and advanced materials with KINTEK’s industry-leading thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet the rigorous demands of 1000°C vacuum annealing and atomic-scale interface engineering.
Whether you are fine-tuning van der Waals gaps or inducing critical phase transformations, our lab high-temp furnaces provide the environmental stability your project requires. Contact us today to discuss your unique needs and see how our expertise can drive your next breakthrough.
Visual Guide
References
- Dae Yeop Jeong, Won Il Park. α‐<scp>Al<sub>2</sub>O<sub>3</sub></scp> Networks on <scp>MLG</scp> Membranes for Continuous Lithium Ion Extraction from Artificial Sea Water with Enhanced Selectivity and Durability. DOI: 10.1002/eem2.70145
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What role does graphite fixturing play in the heat treatment of steel aerospace components? Ensure Precision and Minimize Distortion
- What is the role of insulation materials in a vacuum furnace? Boost Efficiency and Precision in High-Temp Processing
- What are the categories of vacuum sintering furnaces based on vacuum degree? Choose the Right System for Your Materials
- How are vacuum coating furnaces applied in the semiconductor and electronic components industry? Essential for High-Purity Electronics
- What are the core functions of dual-chamber vacuum heat treatment systems? Master Precision Gas Quenching
- How does vacuum brazing benefit the electronics industry? Achieve Superior Thermal Management and Reliability
- What is the purpose of the water cooling system in a vacuum furnace? Ensure Safety and Efficiency in High-Temp Operations
- What role do vacuum annealing furnaces play in optical material processing? Enhance Clarity and Performance for Your Optics



















