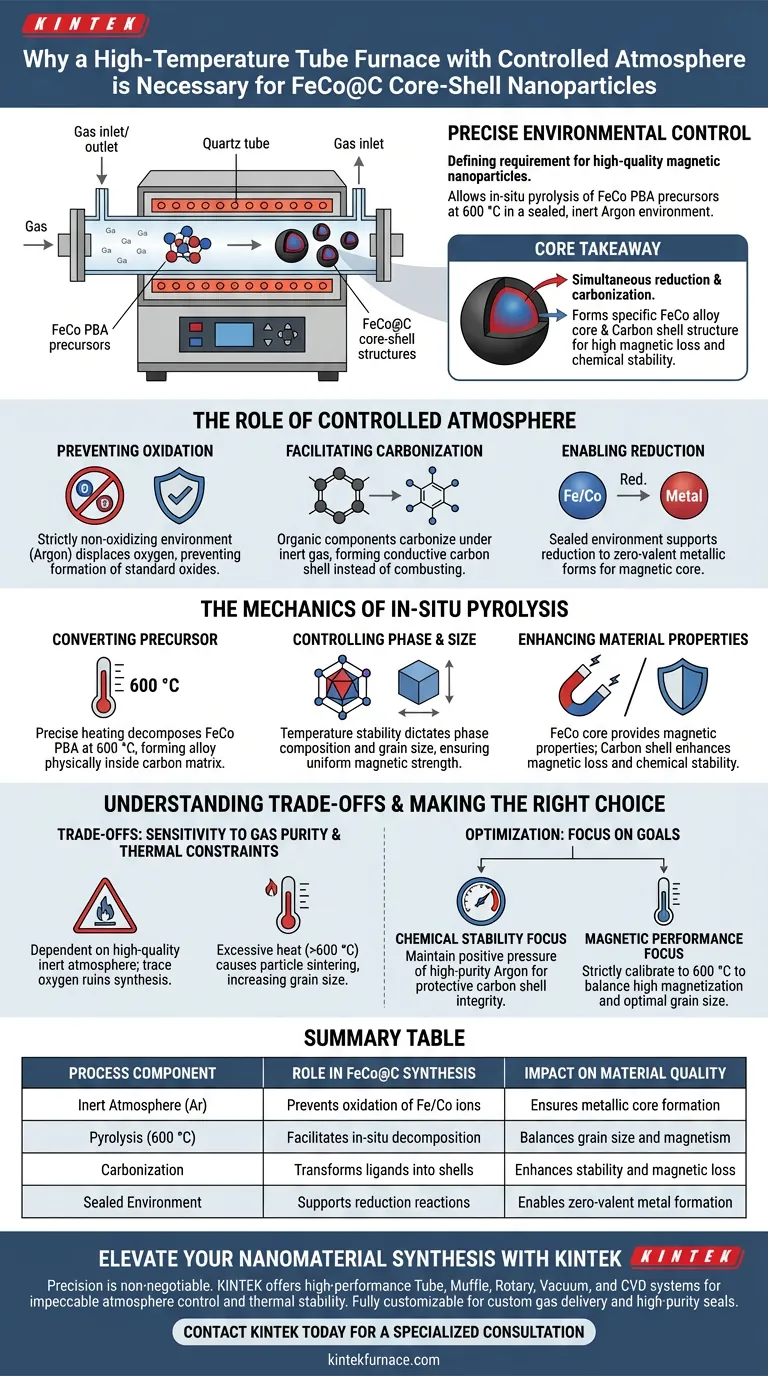
Precise environmental control is the defining requirement for synthesizing high-quality magnetic nanoparticles. A high-temperature tube furnace is necessary because it provides a sealed, inert environment—typically using Argon—that allows for the in-situ pyrolysis of FeCo Prussian Blue Analog (PBA) precursors at 600 °C without oxidizing the metal.
Core Takeaway The tube furnace enables a simultaneous reduction and carbonization process that converts precursors into a specific core-shell structure (FeCo alloy core, Carbon shell). This dual structure is critical for achieving the high magnetic loss and chemical stability required for advanced applications.

The Role of Controlled Atmosphere
Preventing Oxidation
The synthesis of metallic FeCo requires a strictly non-oxidizing environment.
If oxygen is present during heating, the iron and cobalt will form standard oxides rather than the desired metallic alloy. The tube furnace utilizes a protective atmosphere (specifically Argon for FeCo precursors) to displace oxygen entirely.
Facilitating Carbonization
The atmosphere enables the organic components of the precursor to transform rather than burn.
Under inert gas protection, the ligands in the precursor undergo carbonization. This forms a conductive carbon shell around the metal core, rather than combusting into ash as they would in an open-air oven.
Enabling Reduction
The sealed environment supports the reduction of metal ions.
Specific reducing gases released during the decomposition of the carbon source help convert iron and cobalt precursors into their zero-valent metallic forms. This is essential for creating the magnetic core.
The Mechanics of In-Situ Pyrolysis
Converting the Precursor
The furnace uses precise heating programs to break down FeCo Prussian Blue Analog (PBA) precursors.
At a targeted temperature of 600 °C, the precursor thermally decomposes. This "in-situ" process ensures that the metal alloy forms physically inside the developing carbon matrix, ensuring a tight core-shell bond.
Controlling Phase and Size
Temperature stability directly impacts the physical properties of the nanoparticle.
The precise thermal control allows you to dictate the phase composition and grain size of the material. Fluctuations in temperature could lead to uneven grain growth or incomplete phase transformation, compromising magnetic strength.
Enhancing Material Properties
The resulting core-shell structure serves two distinct functions.
The FeCo alloy core provides strong magnetic properties. The conductive carbon shell enhances magnetic loss and protects the metallic core from environmental degradation, ensuring long-term chemical stability.
Understanding the Trade-offs
Sensitivity to Gas Purity
The success of this method is entirely dependent on the quality of the inert atmosphere.
Even trace amounts of oxygen due to leaks or low-grade Argon can ruin the synthesis by oxidizing the FeCo surface. The furnace seals must be impeccable.
Thermal Constraints
While high temperatures are necessary for carbonization, excessive heat has drawbacks.
Temperatures significantly above the optimal 600 °C range may cause particle sintering (agglomeration). This increases grain size undesirably and reduces the specific surface area of the material.
Making the Right Choice for Your Goal
To optimize your synthesis of FeCo@C nanoparticles, align your furnace settings with your specific objectives:
- If your primary focus is Chemical Stability: Ensure your system maintains a positive pressure of high-purity Argon to maximize the integrity of the protective carbon shell.
- If your primary focus is Magnetic Performance: Strictly calibrate your temperature program to 600 °C to balance high magnetization with optimal grain size, avoiding over-sintering.
By strictly controlling the pyrolysis environment, you transform a delicate precursor into a robust, high-performance magnetic composite.
Summary Table:
| Process Component | Role in FeCo@C Synthesis | Impact on Material Quality |
|---|---|---|
| Inert Atmosphere (Ar) | Prevents oxidation of Fe/Co ions | Ensures metallic core formation |
| Pyrolysis (600 °C) | Facilitates in-situ decomposition | Balances grain size and magnetism |
| Carbonization | Transforms ligands into shells | Enhances stability and magnetic loss |
| Sealed Environment | Supports reduction reactions | Enables zero-valent metal formation |
Elevate Your Nanomaterial Synthesis with KINTEK
Precision is non-negotiable when synthesizing delicate core-shell structures like FeCo@C. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems designed to provide the impeccable atmosphere control and thermal stability your research demands.
Whether you require custom gas delivery or high-purity seals for sensitive pyrolysis, our lab high-temperature furnaces are fully customizable to meet your unique specifications.
Ready to achieve superior magnetic performance and chemical stability?
Contact KINTEK Today for a Specialized Consultation
Visual Guide
References
- Zhuomin Jiang, Kangwon Lee. Multifunctional Ultrathin Recycled PET‐Based Membrane for Electromagnetic Interference Shielding, Antibacterial and Thermal Management. DOI: 10.1002/admi.202301047
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What are the benefits of using multiple diameter tube options in a vertical tube furnace? Boost Lab Versatility and Efficiency
- What special features does the quartz tube furnace have for sample handling? Unlock Visibility and Purity in High-Temp Processes
- What conditions does a tube furnace provide for post-ion-implantation? Achieve Precise Microstructural Repair
- How does the temperature zone layout of a horizontal tube furnace affect the synthesis quality of Bi2Se3 nanofilms?
- What role does a laboratory tube furnace system play in the catalytic pyrolysis of LLDPE? Enhancing Yield and Precision
- What role does a vacuum tube furnace play in the preparation of wheat straw biochar? Master Controlled Pyrolysis
- What factors should be considered when procuring a three-zone tube furnace? Ensure Precision and Uniformity for Your Lab
- How does a dual-zone tube furnace control CoTeO4 crystal growth? Precision CVT Thermal Gradient Methods



















