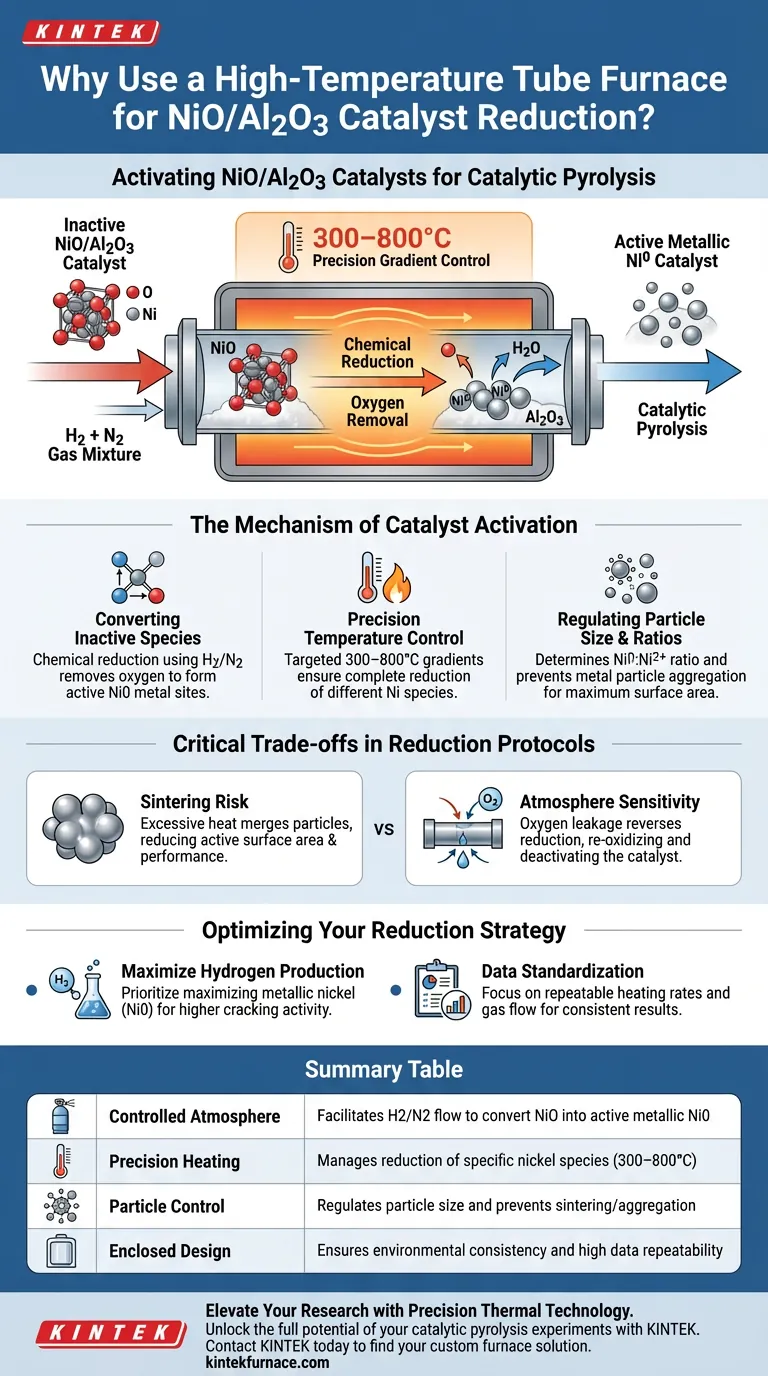
A high-temperature tube furnace is the critical tool used to activate NiO/Al2O3 catalysts by creating a controlled reducing atmosphere. It allows for the precise introduction of a hydrogen and nitrogen gas mixture, which chemically converts inactive nickel oxide species into active metallic nickel (Ni0) prior to pyrolysis.
By enabling the flow of reducing gases and maintaining exact temperature gradients, the tube furnace transforms the catalyst's chemical state. This process determines the ratio of metallic nickel to ionic nickel and controls particle size, two factors that directly dictate the catalyst's cracking activity and hydrogen production efficiency.

The Mechanism of Catalyst Activation
Converting Inactive Species to Active Metal
The primary function of the furnace in this context is chemical reduction. The NiO/Al2O3 catalyst initially exists in an oxide or spinel state, which is not catalytically active for the desired pyrolysis reactions.
By introducing a controlled mixture of hydrogen and nitrogen, the furnace facilitates the removal of oxygen from the nickel compounds. This creates metallic nickel (Ni0), the active site required for effective catalysis.
Precision Temperature Control
The effectiveness of the reduction process relies heavily on the thermal environment. The tube furnace provides precise temperature gradient control, typically ranging from 300 to 800 degrees Celsius.
This specific range is necessary because different nickel species reduce at different temperatures. An uncontrolled thermal environment would result in uneven activation across the catalyst bed.
Regulating Particle Size and Ratios
The thermal profile applied during reduction does more than just remove oxygen; it structures the catalyst's surface. The temperature directly determines the size of the metal particles and the final ratio of Ni0 (metallic) to Ni2+ (ionic) species.
If the temperature is too low, reduction is incomplete. If it is too high or unregulated, metal particles may aggregate, reducing the active surface area.
Ensuring Environmental Consistency
Beyond temperature, the tubular design offers a highly enclosed heating environment. This allows researchers to strictly regulate residence times and heating rates without external interference.
This consistency is vital for generating standardized data. It ensures that the observed cracking activity is a result of the catalyst's properties, not fluctuations in the activation environment.
Critical Trade-offs in Reduction Protocols
Balancing Reduction vs. Sintering
While high temperatures are needed to fully reduce nickel species, excessive heat can lead to sintering. This is when small metal particles merge into larger clusters, drastically reducing the active surface area and lowering catalytic performance.
Atmosphere Sensitivity
The tube furnace allows for a specific gas mixture, but this requires strict management of the anaerobic environment. Any leakage of oxygen during the reduction phase effectively reverses the process, re-oxidizing the nickel and rendering the catalyst inactive before the experiment begins.
Making the Right Choice for Your Goal
## How to Optimize Your Reduction Strategy
The setup of your high-temperature tube furnace should be dictated by the specific outcomes you need from your pyrolysis experiment.
- If your primary focus is maximizing Hydrogen Production: Prioritize a reduction protocol that maximizes the formation of metallic nickel (Ni0), as this directly correlates with higher cracking activity.
- If your primary focus is Data Standardization: Focus on the repeatability of heating rates and gas flow, ensuring that the environmental parameters remain identical across every experimental run to minimize interference.
The success of your catalytic pyrolysis depends less on the catalyst you buy, and more on how precisely you activate it within the furnace.
Summary Table:
| Feature | Impact on Catalyst Activation |
|---|---|
| Controlled Atmosphere | Facilitates H2/N2 flow to convert NiO into active metallic Ni0 |
| Precision Heating | Manages reduction of specific nickel species (300–800°C) |
| Particle Control | Regulates particle size and prevents sintering/aggregation |
| Enclosed Design | Ensures environmental consistency and high data repeatability |
Elevate Your Research with Precision Thermal Technology
Unlock the full potential of your catalytic pyrolysis experiments with KINTEK. As a leader in expert R&D and manufacturing, we provide high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems tailored to your laboratory's exact specifications. Our customizable high-temperature furnaces ensure the precise temperature gradients and controlled atmospheres necessary to maximize your catalyst's metallic nickel formation and hydrogen production efficiency.
Don't let inconsistent activation compromise your data. Contact KINTEK today to find your custom furnace solution and achieve superior material performance.
Visual Guide
References
- Bo Zhang, Xiang Li. Catalytic Pyrolysis of Waste Textiles for Hydrogen-Rich Syngas Production over NiO/Al2O3 Catalyst. DOI: 10.3390/pr13010015
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What are the key features of tube furnaces? Unlock Precision in High-Temperature Processing
- How does a vacuum tube furnace support the sintering process of np-CuSn films? Achieve High-Purity Intermetallic Joints
- How does tube furnace cracking compare to fuel furnaces in terms of efficiency? Discover Higher Efficiency and Precision
- What are the different types of tube furnaces and their features? Choose the Right Furnace for Your Lab
- Why is a high-temperature tube furnace used for AlPO4 calcination? Ensure Safety in Molten Salt Electrolysis
- What technical features make a laboratory horizontal tube furnace an ideal reaction device for oil sludge studies?
- What is the function of a dual-temperature zone tube furnace in CVD? Enhance MoS2/GaN Synthesis Precision
- What is the function of a Quartz Tube Furnace in the dry thermal oxidation of silicon wafers? Enhance Your Oxide Quality



















