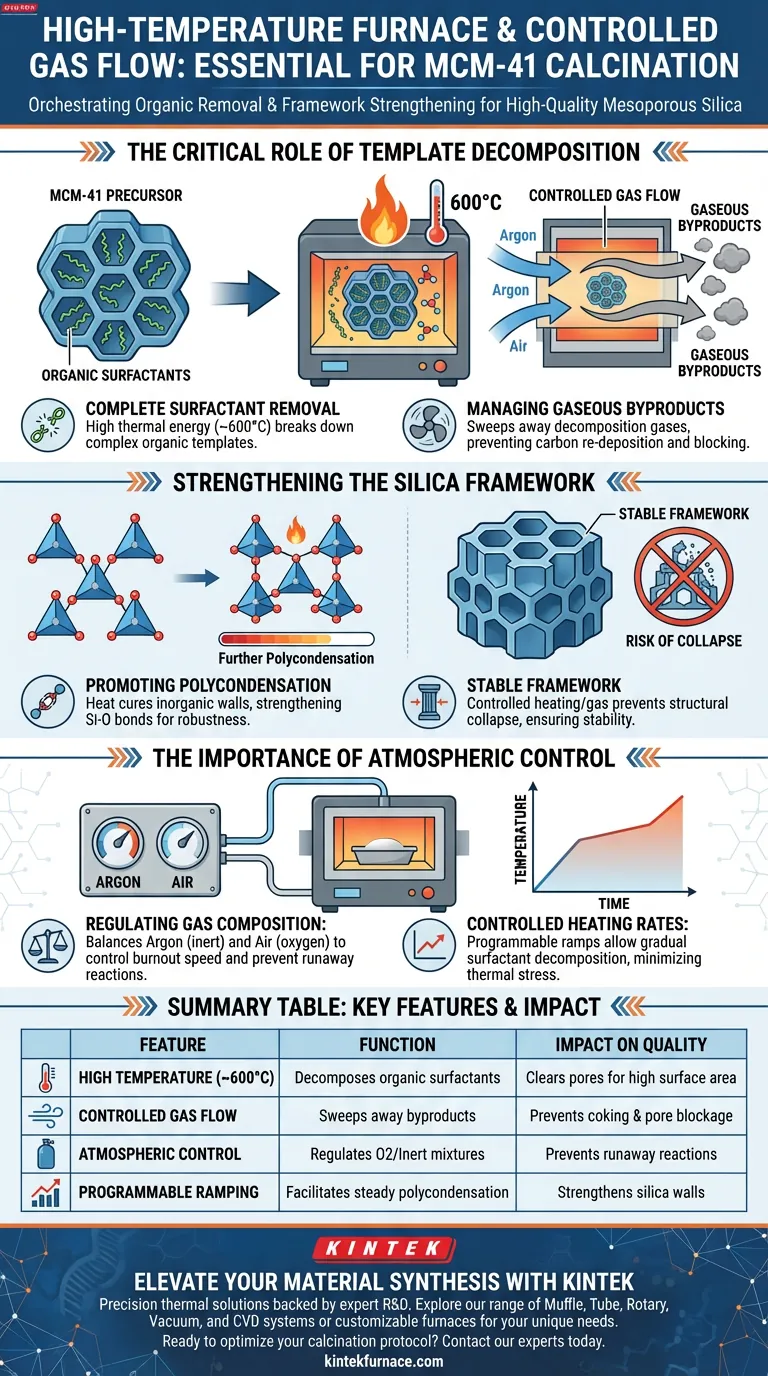
A high-temperature furnace with controlled gas flow is essential for the successful synthesis of MCM-41 because it orchestrates the delicate removal of organic templates without destroying the material's structure. This specific equipment allows for precise heating around 600 °C and the regulation of gas atmospheres, such as argon and air mixtures, to decompose surfactants into gaseous products efficiently.
Calcination is a dual-process of destruction and reinforcement. The controlled environment ensures organic templates are completely removed while simultaneously driving the polycondensation required to lock the silica framework into a stable, porous structure.
The Critical Role of Template Decomposition
Complete Surfactant Removal
MCM-41 is synthesized using organic surfactants that act as a mold for its pores. To make the material useful, these organic templates must be fully eliminated.
The furnace provides the necessary thermal energy, typically reaching 600 °C, to break down these complex organic molecules. Without this high heat, the pores would remain blocked, rendering the material useless for adsorption or catalysis.
Managing Gaseous Byproducts
As the surfactants decompose, they transform into gaseous products. A static oven cannot effectively manage this transition.
Controlled gas flow is required to physically sweep these gases away from the material. This prevents the re-deposition of carbon residues and ensures the pores are left clean and accessible.
Strengthening the Silica Framework
Promoting Polycondensation
Heat does more than just burn off the template; it cures the inorganic walls of the material. The calcination process drives further polycondensation of the silica framework.
This chemical reaction strengthens the bonds between silicon and oxygen atoms. It creates a robust, cross-linked structure capable of withstanding physical stress.
Preventing Structural Collapse
The greatest risk during calcination is the collapse of the delicate pore walls. If the template is removed before the walls are fully strengthened, the structure will crumble.
By strictly controlling the heating rate and gas composition, the furnace creates an environment where the framework hardens before or during the removal of the support template, ensuring structural stability.
The Importance of Atmospheric Control
Regulating Gas Composition
The primary reference highlights the use of specific mixtures, such as argon and air. This capability is distinct from a standard air-only oven.
Argon can provide an inert buffer, while air provides the oxygen necessary for combustion. Balancing these gases allows you to control the speed and intensity of the template burnout, preventing "runaway" exothermic reactions that could damage the sample.
Controlled Heating Rates
Precision furnaces allow for programmable temperature ramps. This is critical for MCM-41.
A slow, controlled ramp allows the surfactant to decompose gradually. This minimizes thermal stress on the silica framework, further preventing cracks or pore collapse.
Understanding the Trade-offs
The Risk of Inadequate Flow
If the gas flow is too low, organic byproducts may not be flushed out effectively. This often results in a material that is grey or black due to carbon coking, rather than the desired white powder.
Balancing Time and Temperature
While high temperatures are needed, excessive heat or prolonged exposure can lead to sintering. This causes the pore walls to densify too much, potentially shrinking the pore size or reducing the overall surface area.
Optimizing Your Calcination Protocol
To ensure high-quality MCM-41, tailor your furnace settings to your specific requirements:
- If your primary focus is Maximum Purity: Ensure sufficient oxygen flow (via air mixture) to facilitate the complete oxidation of all organic surfactants into gas.
- If your primary focus is Structural Integrity: Prioritize a slower heating rate and a balanced gas mixture to prevent thermal shock and support steady polycondensation.
Success lies in using the furnace not just as a heater, but as a tool to precisely synchronize organic decomposition with inorganic strengthening.
Summary Table:
| Feature | Function in MCM-41 Calcination | Impact on Material Quality |
|---|---|---|
| High Temperature (~600°C) | Decomposes organic surfactants/templates | Clears pores for high surface area |
| Controlled Gas Flow | Sweeps away gaseous byproducts/carbon residues | Prevents coking and pore blockage |
| Atmospheric Control | Regulates O2/Inert gas (Argon) mixtures | Prevents runaway exothermic reactions |
| Programmable Ramping | Facilitates steady polycondensation | Strengthens silica walls and prevents collapse |
Elevate Your Material Synthesis with KINTEK
Precision is non-negotiable when synthesizing delicate structures like MCM-41. KINTEK provides industry-leading thermal solutions backed by expert R&D and manufacturing to ensure your research yields consistent, high-purity results.
Our specialized range of Muffle, Tube, Rotary, Vacuum, and CVD systems offers the precise atmospheric control and programmable heating ramps required to synchronize organic decomposition with framework strengthening. Whether you need a standard setup or a customizable high-temp furnace tailored to your unique lab requirements, KINTEK delivers the reliability your work deserves.
Ready to optimize your calcination protocol? Contact our experts today to find the perfect furnace for your laboratory needs.
Visual Guide
References
- Michael Karl, Simone Pokrant. Porous MCM‐41 Silica Materials as Scaffolds for Silicon‐based Lithium‐ion Battery Anodes. DOI: 10.1002/celc.202300707
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is the purpose of an atmosphere furnace? Control Gas Environments for Superior Material Processing
- What are metallizing furnaces used for? Bond Metal to Ceramic for Advanced Electronics
- What safety considerations are important when operating atmosphere furnaces? Ensure Explosion-Free Operation with Expert Tips
- What role do inert atmosphere furnaces play in the semiconductor industry? Essential for Purity and Yield
- Why are inert ovens important in electronics manufacturing? Prevent Oxidation and Boost Component Reliability
- What materials and processes are suitable for box type atmosphere furnaces? Versatile Solutions for Controlled Heat Treatment
- Which industries commonly use inert ovens? Essential for Electronics, Metallurgy, and Materials Science
- What is a vacuum atmosphere furnace? Master High-Purity Heat Treatment for Superior Materials



















