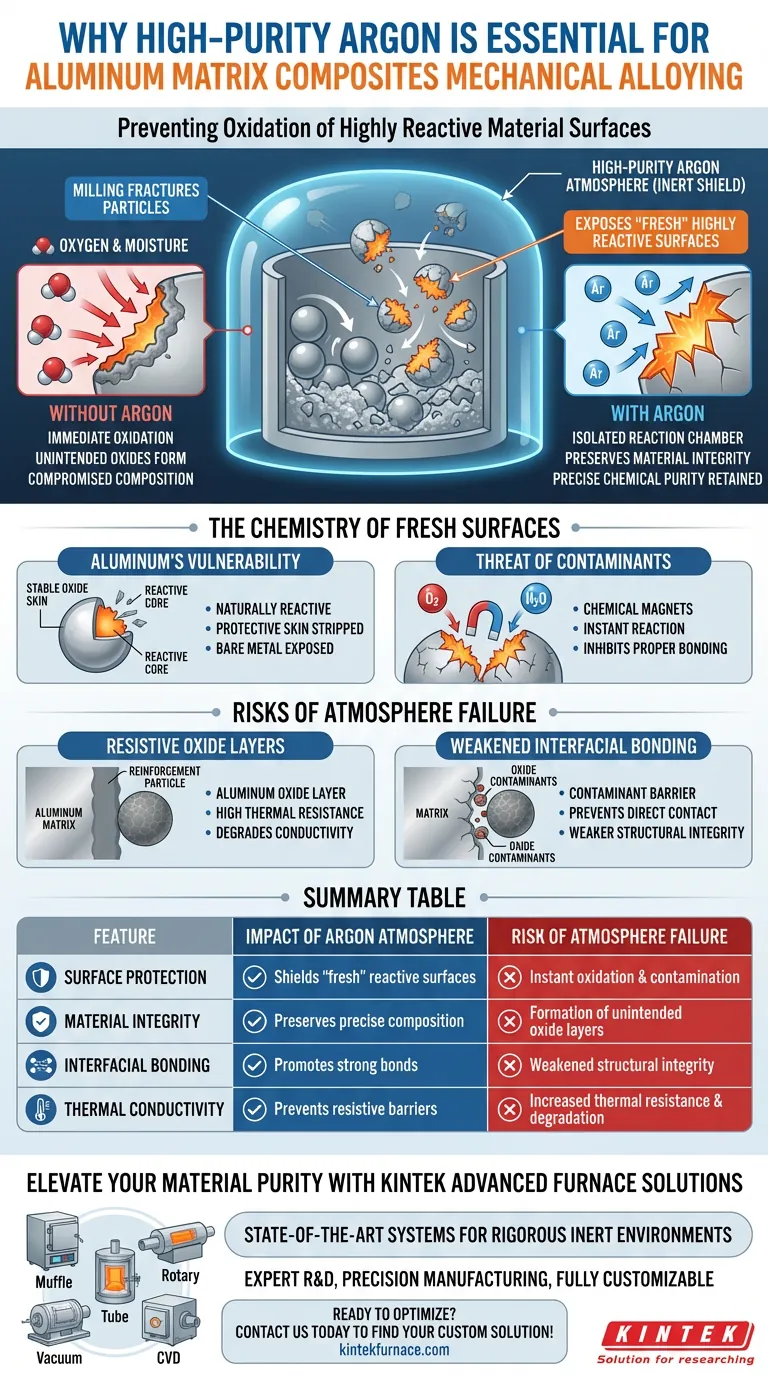
Preventing oxidation of highly reactive material surfaces is the sole purpose of using a high-purity argon atmosphere. During mechanical alloying, the milling process aggressively fractures particles, exposing "fresh" surfaces on the aluminum and reinforcements that are chemically unstable. The argon environment acts as an inert shield, isolating these raw materials from atmospheric oxygen and moisture to maintain chemical purity.
Mechanical alloying fractures particles to expose raw, highly reactive surfaces. Without an inert argon barrier, these fresh surfaces immediately react with environmental oxygen, compromising the composite's chemical composition and inhibiting proper bonding between the matrix and reinforcements.
The Chemistry of Fresh Surfaces
Why Aluminum Powder is Vulnerable
Aluminum is naturally highly reactive with oxygen. In a static state, it is usually protected by a thin, stable oxide skin.
However, mechanical alloying involves high-energy ball milling that constantly fractures particles. This process strips away existing protective layers and exposes bare, unoxidized metal to the environment.
The Threat of Environmental Contaminants
These newly exposed surfaces function like chemical magnets. They will instantly react with moisture or oxygen present in standard air.
If this reaction occurs, the aluminum creates unintended oxides rather than bonding with the intended reinforcement materials, such as TiO2.
The Role of the Argon Shield
Isolating the Reaction Chamber
High-purity argon provides a stable, inert atmosphere that replaces standard air within the milling container.
Because argon does not react chemically with aluminum or ceramic reinforcements, it creates a "safe zone" for the mechanical alloying process.
Preserving Material Integrity
The primary goal is to ensure that the composite consists only of the intended aluminum matrix and reinforcements.
By excluding oxygen and moisture, argon ensures the final powder mixture retains the precise chemical purity required for the material's specific engineering application.
Understanding the Risks of Atmosphere Failure
Formation of Resistive Oxide Layers
If the argon atmosphere is compromised or not sufficiently pure, an aluminum oxide layer will form at the interface of the materials.
While the supplementary data highlights this in the context of sintering, the principle applies here: oxidation creates a barrier. This barrier often possesses high thermal resistance, which can degrade the thermal conductivity of the final composite.
Weakened Interfacial Bonding
A composite relies on strong bonds between the aluminum matrix and the reinforcement particles.
Oxidation acts as a contaminant at these interfaces. This prevents direct contact between the matrix and the reinforcement, potentially leading to weaker structural integrity and reduced performance.
Making the Right Choice for Your Goal
Achieving a high-performance aluminum matrix composite requires strict atmosphere control. Use these guidelines to prioritize your process setup:
- If your primary focus is Chemical Purity: Ensure your milling equipment is perfectly sealed and purged with high-purity argon to prevent moisture from degrading fresh fracture surfaces.
- If your primary focus is Thermal Performance: rigorous atmosphere control is required to prevent the formation of insulating oxide layers that block heat transfer at particle interfaces.
Success in mechanical alloying depends not just on the energy of the mill, but on the purity of the environment in which that energy is applied.
Summary Table:
| Feature | Impact of Argon Atmosphere | Risk of Atmosphere Failure |
|---|---|---|
| Surface Protection | Shields 'fresh' reactive surfaces from air | Instant oxidation and contamination |
| Material Integrity | Preserves precise chemical composition | Formation of unintended oxide layers |
| Interfacial Bonding | Promotes strong matrix-reinforcement bonds | Weakened structural integrity |
| Thermal Conductivity | Prevents formation of resistive barriers | Increased thermal resistance and degradation |
Elevate Your Material Purity with KINTEK Advanced Furnace Solutions
Precise atmosphere control is the difference between a high-performance composite and a failed experiment. KINTEK empowers your research and production with state-of-the-art Muffle, Tube, Rotary, Vacuum, and CVD systems, all designed to maintain the rigorous inert environments your materials demand.
Backed by expert R&D and precision manufacturing, our high-temperature lab furnaces are fully customizable to meet your unique mechanical alloying and sintering requirements. Don't let oxidation compromise your innovation—ensure chemical purity and superior interfacial bonding with our industry-leading thermal technology.
Ready to optimize your alloying process? Contact us today to find your custom solution!
Visual Guide
References
- Chen Wang, Zhiping Sun. Microstructures and Mechanical Properties of Al Matrix Composites Reinforced with TiO2 and Graphitic Carbon Nitride. DOI: 10.3390/met15010060
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Magnesium Extraction and Purification Condensing Tube Furnace
People Also Ask
- Why is atmospheric protection and composition compensation required for Mn-Cu alloys? Master High-Temp Precision
- Why is a high-purity argon atmosphere critical for successful molten salt electrochemical testing? Expert Guide
- Why is it necessary to use an atmosphere furnace for MOF melt-quenching? Protect Fragile Materials from Decomposition
- What is the function of an air annealing furnace? Enhance Ho:Y2O3 Ceramics Transparency and Performance
- What are the advantages of using a controlled atmosphere furnace? Achieve Precise Material Processing and Quality
- What are the limitations of low vacuum atmosphere furnaces? Understand Trade-offs for Cost-Effective Heat Treatment
- How does a precision high-temperature furnace ensure the densification of MgO? Master Low-Temp Ceramic Sintering
- Why is high-purity nitrogen introduced into the TGA furnace during moxa floss studies? Ensure Precise Thermal Analysis



















