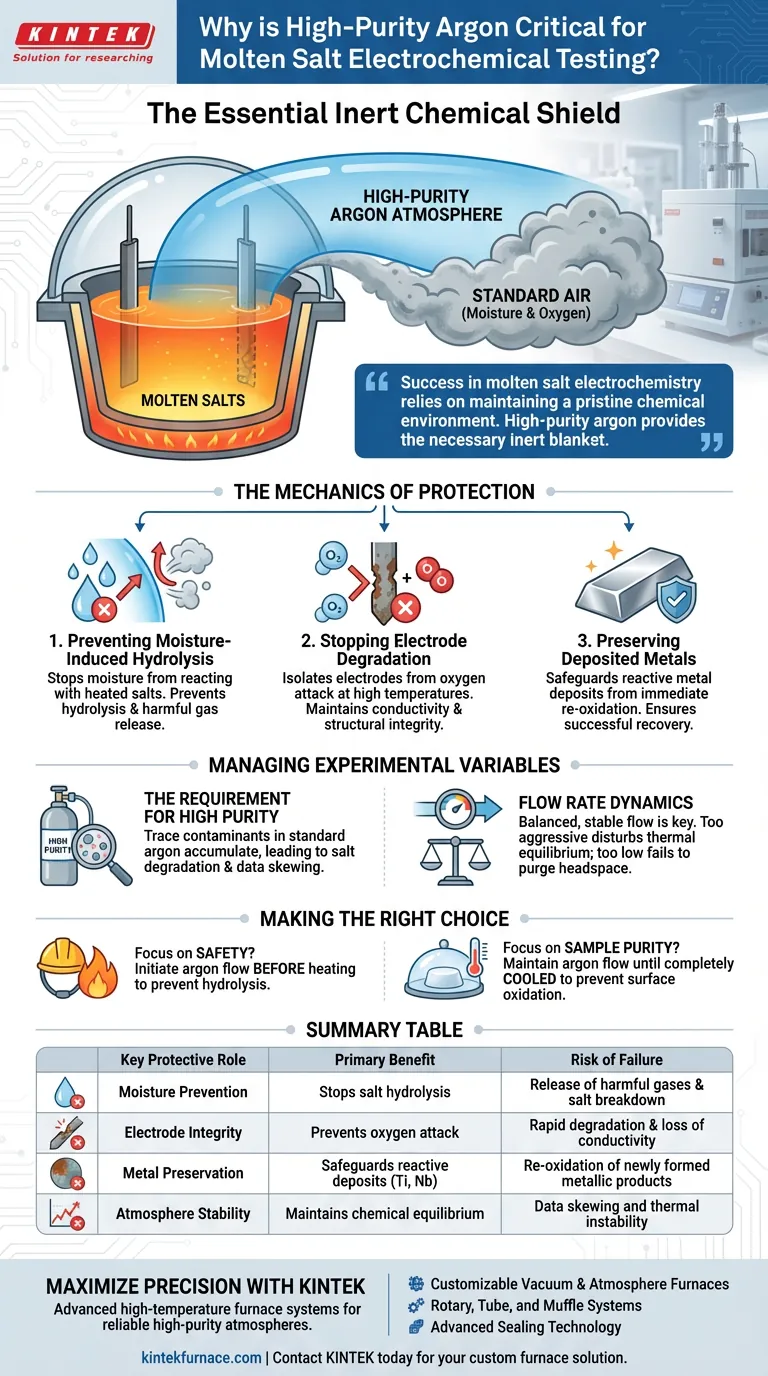
A high-purity argon atmosphere acts as an essential chemical shield for molten salt experiments. It displaces standard air to prevent moisture and oxygen from chemically attacking the molten salts and the reactive metals being processed. Without this inert barrier, the integrity of the electrochemical process is immediately compromised by uncontrolled side reactions.
Success in molten salt electrochemistry relies on maintaining a pristine chemical environment. High-purity argon provides the necessary inert blanket that prevents dangerous hydrolysis of salts and safeguards sensitive electrode materials from oxidation.

The Mechanics of Protection
Preventing Moisture-Induced Hydrolysis
Molten salts are highly susceptible to reacting with ambient humidity found in standard air. When moisture contacts the heated salt bath, a chemical reaction known as hydrolysis occurs.
This reaction effectively breaks down the salt components. More critically, the primary reference notes that this process can produce harmful gases, posing a safety risk to the operator and altering the fundamental chemistry of the electrolyte.
Stopping Electrode Degradation
Oxygen is highly reactive at the elevated temperatures required for electrolysis. Without a protective atmosphere, oxygen will rapidly attack and degrade the electrode materials.
A stable argon flow isolates the system. This prevents the electrodes from oxidizing, ensuring they maintain their conductivity and structural integrity throughout the duration of the test.
Preserving Deposited Metals
In many electrochemical tests, the goal is to deposit reactive metals, such as niobium and titanium. These metals typically have a high affinity for oxygen.
If exposed to air while hot, these newly formed metallic deposits will immediately re-oxidize. The argon atmosphere preserves the reduction work performed during the experiment, allowing the pure metal to be recovered successfully.
Managing Experimental Variables
The Requirement for High Purity
The term "high-purity" is not merely a suggestion; it is a functional requirement. Standard industrial argon may contain trace amounts of moisture or oxygen.
In these sensitive systems, even trace contaminants can accumulate over time. This leads to gradual degradation of the salt bath or slight oxidation of the product, skewing experimental data.
Flow Rate Dynamics
The primary reference emphasizes a "stable flow" of argon. This flow must be consistent enough to continuously purge the headspace of the reaction vessel.
However, users must balance this flow. If the flow is too aggressive, it could potentially disturb the thermal equilibrium of the melt or increase the evaporation rate of volatile salt components.
Making the Right Choice for Your Goal
To ensure the validity of your electrochemical testing, consider the following applications of the argon atmosphere:
- If your primary focus is Safety: Initiate the argon flow before heating the salts to prevent hydrolysis and the subsequent release of harmful gases.
- If your primary focus is Sample Purity: Maintain the high-purity argon flow until the system has completely cooled to room temperature to prevent surface oxidation of niobium or titanium deposits.
By rigorously controlling the atmosphere with argon, you transform a potentially volatile reaction into a precise, reproducible scientific process.
Summary Table:
| Key Protective Role | Primary Benefit | Risk of Failure |
|---|---|---|
| Moisture Prevention | Stops salt hydrolysis | Release of harmful gases & salt breakdown |
| Electrode Integrity | Prevents oxygen attack | Rapid degradation & loss of conductivity |
| Metal Preservation | Safeguards reactive deposits (Ti, Nb) | Re-oxidation of newly formed metallic products |
| Atmosphere Stability | Maintains chemical equilibrium | Data skewing and thermal instability |
Maximize Your Experimental Precision with KINTEK
Maintaining a pristine chemical environment is non-negotiable for successful molten salt electrochemistry. KINTEK provides the advanced high-temperature furnace systems required to maintain high-purity atmospheres with absolute reliability.
Backed by expert R&D and manufacturing, we offer:
- Customizable Vacuum & Atmosphere Furnaces designed for inert gas stability.
- Rotary, Tube, and Muffle Systems optimized for reactive metal processing.
- Advanced Sealing Technology to prevent moisture and oxygen ingress.
Don't let trace contaminants compromise your research. Contact KINTEK today to find the perfect customizable furnace solution for your lab's unique high-temperature needs.
Visual Guide
References
- Bo Zhang, Maofa Jiang. Electrochemical Behavior of Niobium Oxide and Titanium Oxide in NaF–Na3AlF6 Molten Salt. DOI: 10.3390/met14030297
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- Why must NaFePO4 carbonization be in an inert atmosphere furnace? Ensure High Conductivity and Material Stability
- What are the common applications of box-type atmosphere furnaces? Essential for High-Temperature Controlled Environments
- Which industries benefit from the versatility of retort furnaces? Unlock Precise Heat and Atmosphere Control
- What provides inert atmosphere for high-temperature metallurgical process? Prevent Oxidation with Argon & More
- How does a precision tempering furnace influence SCM440 steel? Optimize Grain Architecture & Nitriding Prep
- Why are retort furnaces significant in industrial applications? Unlock Precision Heat Treatment and Superior Material Quality
- What are the structural characteristics of an atmosphere box furnace? Key Features for Controlled Environments
- When are Controlled Atmosphere Muffle Furnaces typically required? Essential for High-Purity Heat Treatment



















