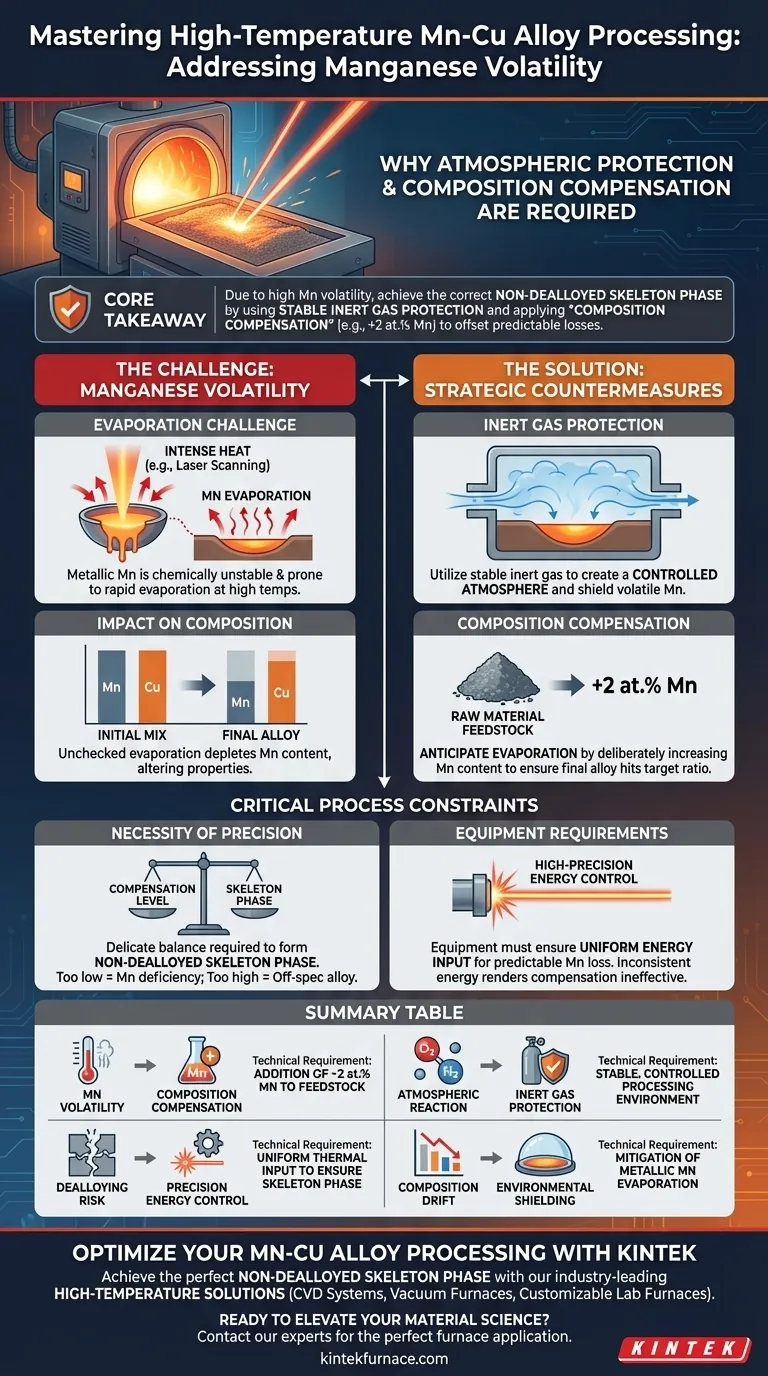
High-temperature processing of Manganese-Cu alloys requires strict environmental and chemical controls due to the extreme volatility of metallic manganese. When subjected to intense heat sources like laser scanning, manganese evaporates rapidly, requiring both a protective inert atmosphere and a deliberate surplus of Mn in the starting material to ensure the final alloy meets design specifications.
Core Takeaway Because metallic manganese has a high propensity for evaporation during thermal processing, standard feedstock ratios result in a manganese-deficient final product. To achieve the correct non-dealloyed skeleton phase, manufacturers must utilize stable inert gas protection and apply "composition compensation"—typically adding an extra 2 at.% Mn to the initial mix—to offset these predictable losses.

Understanding Manganese Volatility
The Evaporation Challenge
During high-temperature processes such as laser scanning, the thermal energy applied to the material is intense.
Under these conditions, metallic manganese (Mn) is chemically unstable and highly prone to evaporation. This volatility creates an immediate risk of material loss during the melting phase.
The Impact on Composition
If this evaporation is left unchecked, the final chemical composition of the alloy will drift away from the intended design.
This loss is not uniform; it specifically depletes the manganese content relative to the copper, fundamentally altering the alloy's properties and potential performance.
Strategic Countermeasures
Inert Gas Protection
To mitigate environmental instability, the processing equipment must utilize stable inert gas protection.
This creates a controlled atmosphere around the melt pool. It shields the volatile manganese from atmospheric reactions and helps stabilize the immediate processing environment.
Composition Compensation
Process engineers must anticipate the evaporation rather than just trying to prevent it. This is achieved through initial composition compensation.
By deliberately increasing the manganese content in the raw material—typically by 2 at.%—manufacturers can "feed" the evaporation process. This ensures that after the inevitable losses occur, the remaining material settles at the exact intended ratio.
Critical Process Constraints
The Necessity of Precision
This is a delicate balancing act. The goal is to form a specific microstructure known as the non-dealloyed skeleton phase.
If the compensation is too low, the skeleton phase will not form correctly due to Mn deficiency. If the compensation is too high (without corresponding evaporation), the alloy will be off-spec.
Equipment Requirements
Material chemistry alone cannot solve the problem. The manufacturing equipment must be capable of high-precision energy control.
Inconsistent energy input can lead to unpredictable evaporation rates, rendering the fixed composition compensation ineffective. Uniform energy application is required to make the Mn loss predictable and manageable.
Ensuring Process Success
To successfully manufacture Mn-Cu alloys via high-temperature methods, prioritize the following based on your production goals:
- If your primary focus is Chemical Accuracy: Implement a standard composition compensation strategy (e.g., +2 at.% Mn) to neutralize the effects of evaporation.
- If your primary focus is Microstructural Integrity: Ensure your equipment offers high-precision energy control to maintain the stable conditions required for the non-dealloyed skeleton phase.
Precision in both atmospheric control and feedstock chemistry is the only way to guarantee the final alloy matches its design requirements.
Summary Table:
| Process Challenge | Solution | Technical Requirement |
|---|---|---|
| Mn Volatility | Composition Compensation | Addition of ~2 at.% Mn to feedstock |
| Atmospheric Reaction | Inert Gas Protection | Stable, controlled processing environment |
| Dealloying Risk | Precision Energy Control | Uniform thermal input to ensure skeleton phase |
| Composition Drift | Environmental Shielding | Mitigation of metallic manganese evaporation |
Optimize Your Mn-Cu Alloy Processing with KINTEK
Don't let manganese volatility compromise your material integrity. Achieving the perfect non-dealloyed skeleton phase requires the highest levels of thermal precision and atmospheric control.
KINTEK provides industry-leading high-temperature solutions—including CVD systems, vacuum furnaces, and customizable lab furnaces—designed to handle the most volatile materials. Backed by expert R&D and manufacturing, our equipment ensures uniform energy input and stable inert environments tailored to your specific research or production needs.
Ready to elevate your material science? Contact our experts today to find the perfect furnace for your specialized application.
Visual Guide
References
- Haozhang Zhong, Ma Qian. Skeletal High‐Strength Nanoporous Copper and Metamaterials: The Hakka Tulou Design Heritage. DOI: 10.1002/adma.202503701
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How is helium utilized in atmosphere furnaces? Unlock Purity and Rapid Cooling for Superior Results
- What are the design configurations of retort furnaces? Optimize Your Thermal Processing with the Right Setup
- How does a controlled atmosphere furnace prevent oxidation and decarburization? Master Precision Heat Treatment
- Why is temperature and atmosphere monitoring critical in furnace operations? Ensure Safety and Quality in Heat Treatment
- What are the advantages of using an inert atmosphere furnace? Achieve Purity, Efficiency, and Safety in Thermal Processing
- What role does an industrial-grade atmospheric furnace play in fire simulation tests? Master ASTM Safety Standards
- What is the purpose of sulfur-enriched environments for MoS2-WS2 heterojunctions? Ensure Optimal Crystal Stoichiometry
- What is the role of calcining beta-zeolite at 750°C? Mastering Phase Transformation for High-Performance Catalysts



















