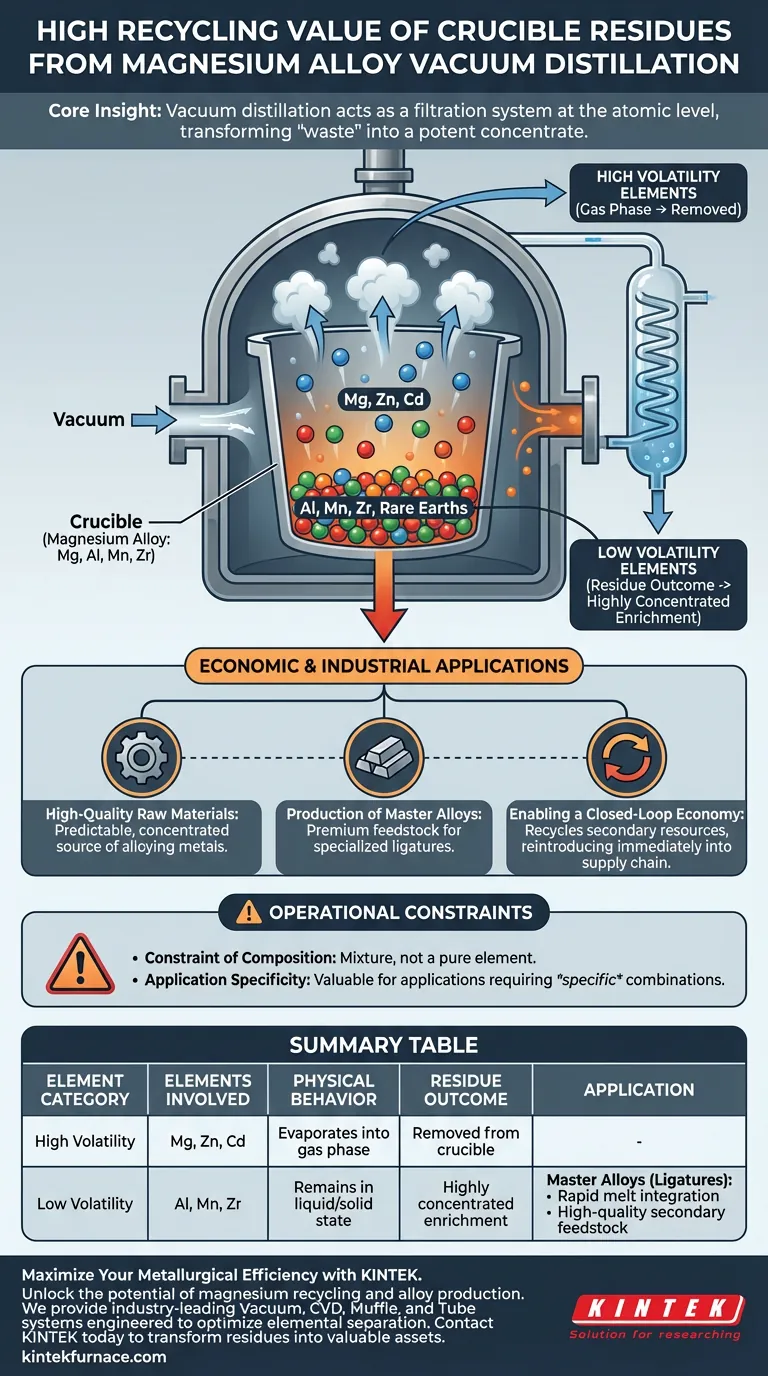
The high recycling value of crucible residues stems from the selective concentration of low-volatility alloying elements. When magnesium alloys undergo vacuum distillation, the volatile magnesium matrix evaporates, leaving behind a chemically enriched byproduct that is chemically superior for downstream manufacturing.
Core Insight: Vacuum distillation acts as a filtration system at the atomic level. By removing the bulk magnesium, the process transforms the remaining "waste" into a potent concentrate of aluminum, manganese, and zirconium, which serves as a premium feedstock for producing master alloys.
The Mechanics of Elemental Separation
Segregation by Volatility
The fundamental principle driving this value is the difference in vapor pressure between elements.
During vacuum distillation, highly volatile elements—specifically magnesium, zinc, and cadmium—transition into the gas phase. These are removed from the crucible and condensed elsewhere.
Enrichment of the Residue
As the volatile elements evaporate, the relative concentration of the remaining materials increases drastically.
Elements with low volatility cannot escape the crucible under these processing conditions.
This results in a residue that is heavily enriched with aluminum, manganese, zirconium, and rare earth elements.
Economic and Industrial Applications
High-Quality Raw Materials
The residue is not a random mixture of scrap; it is a predictable, concentrated source of alloying metals.
Because these elements are already integrated into a metallic matrix, they serve as high-quality raw materials.
Production of Master Alloys
The primary application for these residues is the preparation of specialized magnesium-based master alloys (also known as ligatures).
Master alloys are concentrated mixtures used to introduce specific elements into a melt more quickly and precisely than adding pure metals.
Enabling a Closed-Loop Economy
Utilizing these residues facilitates the recycling of secondary resources.
Instead of discarding the crucible contents or requiring complex chemical extraction, the material is immediately reintroduced into the supply chain, creating a closed-loop system.
Understanding the Operational Constraints
The Constraint of Composition
It is critical to note that the residue is a mixture, not a pure element.
The value of the residue is dependent on the specific combination of elements left behind (e.g., Al mixed with Mn).
Application Specificity
Because the elements are mixed, the residue is valuable only for applications that require that specific combination of alloying agents.
You cannot easily separate the aluminum from the manganese once they are in this enriched residue state without further, likely expensive, processing.
Making the Right Choice for Your Goal
To maximize the value of these residues, align your objectives with the material's properties:
- If your primary focus is Resource Efficiency: View the vacuum distillation process not just as magnesium purification, but as a dual-stream production method that yields both pure magnesium and valuable alloy concentrates.
- If your primary focus is Alloy Manufacturing: Utilize these residues as a cost-effective substitute for virgin master alloys to introduce aluminum, manganese, or zirconium into new melts.
By treating distillation residues as engineered concentrates rather than waste, you unlock a critical pathway for sustainable and cost-effective metallurgy.
Summary Table:
| Element Category | Elements Involved | Physical Behavior | Residue Outcome |
|---|---|---|---|
| High Volatility | Magnesium, Zinc, Cadmium | Evaporates into gas phase | Removed from crucible |
| Low Volatility | Aluminum, Manganese, Zirconium | Remains in liquid/solid state | Highly concentrated enrichment |
| Application | Master Alloys (Ligatures) | Rapid melt integration | High-quality secondary feedstock |
Maximize Your Metallurgical Efficiency with KINTEK
Unlock the full potential of your magnesium recycling and alloy production. At KINTEK, we provide industry-leading laboratory high-temperature furnaces—including Vacuum, CVD, Muffle, and Tube systems—engineered to optimize elemental separation and resource recovery.
Whether you are refining pure metals or creating specialized master alloys, our customizable solutions are backed by expert R&D to meet your unique manufacturing needs. Contact KINTEK today to discover how our advanced thermal technology can transform your distillation residues into valuable assets and streamline your closed-loop production.
Visual Guide
References
- В. Н. Володин, Alexey Trebukhov. On the Problem of the Distillation Separation of Secondary Alloys of Magnesium with Zinc and Magnesium with Cadmium. DOI: 10.3390/met14060671
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- How does vacuum compare to other atmosphere control methods? Achieve Superior Purity and Simplicity
- Why is controlling sulfur levels beneficial for the removal of tin impurities? Optimize Vacuum Steelmaking Efficiency
- What are the primary objectives and challenges of using high-vacuum conditions for EML testing? Master Material Kinetics
- How should a crucible be handled after being heated in a vacuum furnace? Ensure Material Integrity & Accurate Results
- How does the vacuum environment contribute to medical device manufacturing? Ensure Purity and Precision for Patient Safety
- How are vacuum annealing furnaces applied in scientific research and academic fields? Unlock Material Purity and Precision
- How does heat transfer occur in a high-temperature vacuum furnace, and what factors influence its efficiency? Master Radiant Heat Control
- What is the purpose of using a laboratory vacuum drying oven for post-processing lignin nanofiber membranes?



















