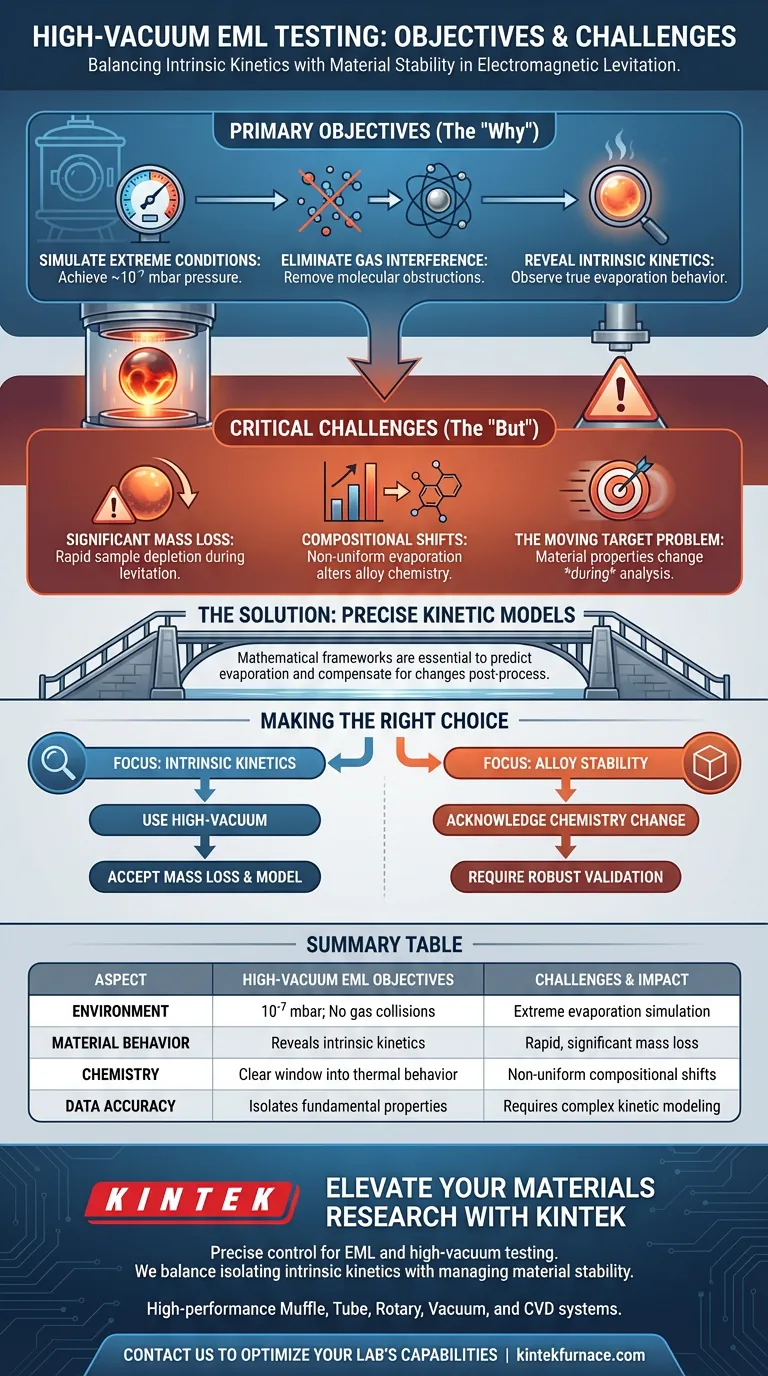
The primary objective of using high-vacuum conditions in Electromagnetic Levitation (EML) testing is to isolate the intrinsic evaporation kinetics of materials by removing environmental interference. However, this creates a critical challenge: rapid mass loss causes significant shifts in alloy composition, necessitating the use of advanced modeling to maintain data accuracy.
High-vacuum environments (typically 10^-7 mbar) eliminate gas collisions to reveal how metals behave under extreme evaporation. The central trade-off is that this process actively alters the material's chemistry during the test, making precise kinetic models essential for valid results.
Achieving Intrinsic Evaporation Environments
Simulating Extreme Conditions
The use of high-vacuum conditions allows researchers to lower pressures to approximately 10^-7 mbar. This creates an environment specifically designed to simulate extreme evaporation scenarios that cannot be replicated under standard atmospheric pressures.
Eliminating Gas Interference
The fundamental advantage of this environment is the elimination of gas molecule collisions. In higher pressure settings, gas molecules obstruct the path of metal atoms, obscuring the true behavior of the material.
Studying Intrinsic Kinetics
By removing these obstructions, researchers can observe the intrinsic evaporation kinetics of the metal. This provides a clear window into how the material loses mass and behaves thermally when external resistance is removed.
The Challenge of Material Stability
Significant Mass Loss
The direct consequence of an unhindered evaporation environment is rapid material depletion. The sample experiences significant mass loss throughout the levitation process, effectively shrinking the specimen as data is collected.
Compositional Shifts
Mass loss is rarely uniform across all elements in an alloy. As specific elements evaporate faster than others, the test induces compositional shifts, changing the chemical makeup of the alloy in real-time.
The Moving Target Problem
This creates a difficult testing variable: the material being analyzed at the end of the process is chemically different from the material at the start. Standard measurement techniques may fail if they assume a static chemical composition.
Understanding the Trade-offs
Necessity of Kinetic Models
To navigate the instability of the sample, researchers must rely on precise kinetic models. These mathematical frameworks are required to predict the rate of evaporation and the resulting changes in chemistry.
Compensating for Elemental Changes
The data collected during high-vacuum EML testing must be corrected post-process. The models allow researchers to compensate for elemental changes, ensuring that the results reflect the properties of the intended alloy rather than the evaporation artifacts.
Making the Right Choice for Your Goal
When designing an EML experiment, consider your specific analytical needs:
- If your primary focus is Intrinsic Kinetics: Utilize high-vacuum conditions to eliminate gas interference, accepting that the sample mass will decrease.
- If your primary focus is Alloy Stability: Recognize that high-vacuum testing alters the sample's chemistry and requires robust modeling to validate the data.
Success in high-vacuum EML testing lies not just in observing the material, but in mathematically predicting how it changes during the observation.
Summary Table:
| Aspect | High-Vacuum EML Objectives | Challenges & Impact |
|---|---|---|
| Environment | Pressure at 10^-7 mbar; eliminates gas collisions | Extreme evaporation simulation |
| Material Behavior | Reveals intrinsic evaporation kinetics | Significant, rapid mass loss |
| Chemistry | Provides a clear window into thermal behavior | Non-uniform compositional shifts |
| Data Accuracy | Isolates fundamental material properties | Requires complex kinetic modeling |
Elevate Your Materials Research with KINTEK
Precise control over extreme conditions is essential for mastering Electromagnetic Levitation (EML) and high-vacuum testing. At KINTEK, we understand the delicate balance between isolating intrinsic kinetics and managing material stability.
Backed by expert R&D and world-class manufacturing, we provide high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you are studying alloy composition shifts or extreme thermal behavior, our lab high-temp furnaces are fully customizable to meet your unique research needs.
Ready to achieve superior data accuracy? Contact us today to discuss how our specialized equipment can optimize your laboratory's testing capabilities.
Visual Guide
References
- Jannatun Nawer, Douglas M. Matson. Thermodynamic assessment of evaporation during molten steel testing onboard the International Space Station. DOI: 10.1038/s41526-024-00416-1
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What is the significance of high-temperature vacuum drying systems for regenerating dehydration materials? Boost Efficiency
- What role does the vacuum system play in the vacuum sintering process? Enhance Glass-Ceramic Density and Strength
- What are the main components of a drop-bottom quench furnace? Essential Parts for Rapid Heat Treatment
- What are the two primary configurations of vacuum furnaces? Hot Wall vs. Cold Wall Explained
- What role do vacuum pumping systems play in vacuum furnaces? Ensure Purity and Control in Thermal Processes
- 1200°C Annealing for LPBF Silicon Steel (Fe-Si): Enhancing Soft Magnetic Performance
- What is the necessity of using a vacuum drying oven for the 70 °C treatment of synthesized TF-COF? Essential Guide
- Why is a high-vacuum environment necessary in copper slag impoverishment? Maximize Your Matte Separation Efficiency



















