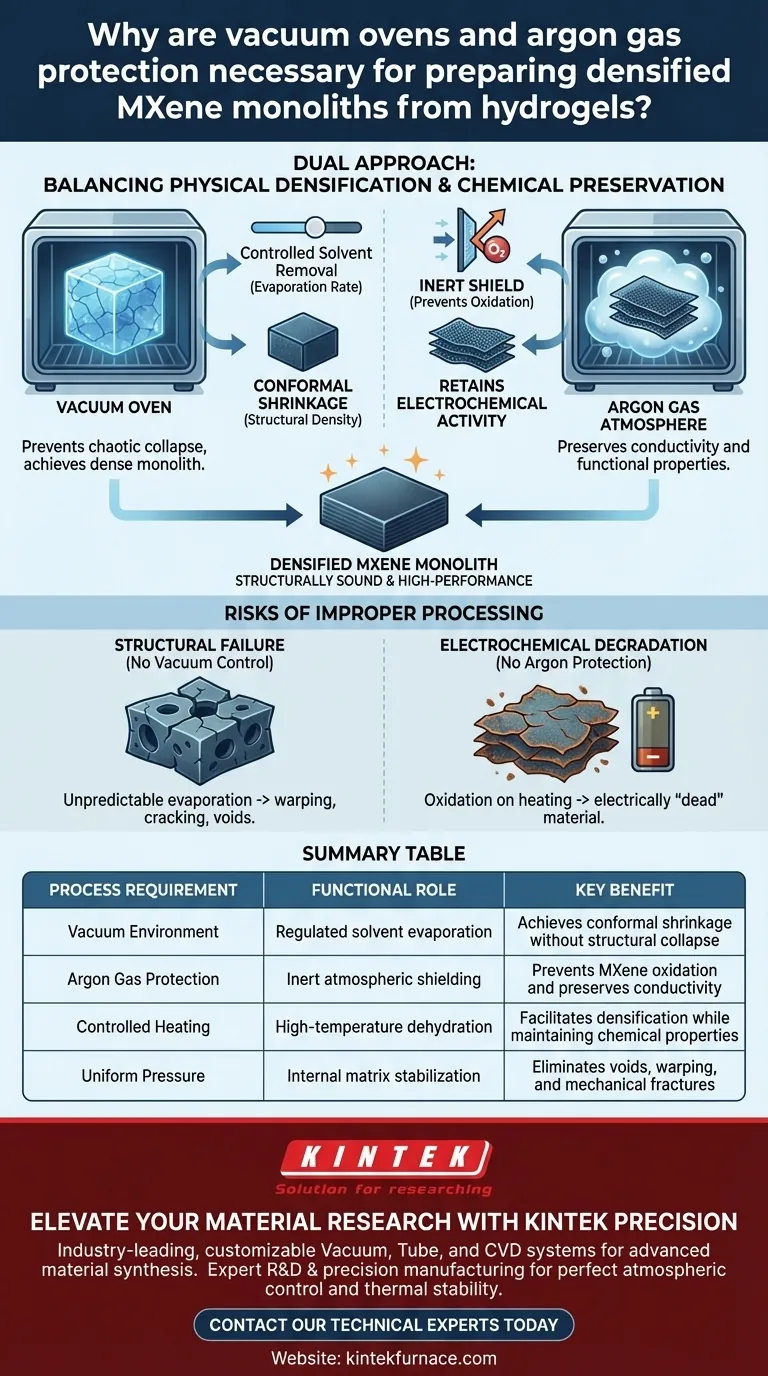
Vacuum ovens and argon gas protection are strictly necessary to balance the physical densification of the material with its chemical preservation. While the vacuum oven regulates the physical removal of solvents to ensure the hydrogel shrinks uniformly, the argon gas provides a chemical shield that prevents the sensitive MXene nanosheets from degrading during the process.
By combining controlled evaporation with an inert atmosphere, you ensure the hydrogel densifies physically without oxidizing chemically. This dual approach is the only way to produce a structurally sound monolith that retains the high electrochemical activity required for high-performance applications.

Controlling Physical Structure via Evaporation
To create a functional monolith, you must transition from a wet hydrogel to a dense solid without destroying the material's architecture.
Regulating the Solvent Removal
The vacuum oven is not used simply to dry the material, but to precisely control the evaporation rate of the solvents.
If solvents evaporate too quickly or unevenly, the internal structure of the hydrogel can collapse chaotically. The vacuum environment allows you to modulate the pressure, ensuring the solvent leaves the matrix at a steady, controlled pace.
Achieving Conformal Shrinkage
The ultimate goal of this controlled evaporation is conformal shrinkage.
As the solvent is removed under vacuum, the hydrogel contracts uniformly. This process, known as densification, transforms the loose hydrogel network into a compact, solid monolith. Without the vacuum control, you would likely end up with a porous or fractured material rather than a densified one.
Preserving Chemical Integrity
MXene nanosheets are highly reactive, particularly when subjected to the heat required for dehydration.
The Threat of Oxidation
Dehydrating a hydrogel generally requires elevated temperatures. In a standard atmosphere, heating MXene triggers a reaction with oxygen.
This oxidation is destructive. It fundamentally alters the chemical structure of the nanosheets, degrading the specific properties—such as conductivity and capacitance—that make MXene valuable.
The Argon Shield
Argon gas is introduced to create an inert environment.
Because argon is chemically non-reactive, it displaces oxygen within the oven. This ensures that even during high-temperature dehydration, the MXene nanosheets have no oxygen to react with. This protection is critical for retaining the material's electrochemical activity in the final monolith.
The Risks of Improper Processing
Understanding what happens when these controls fail highlights their importance.
Structural Failure
Without the vacuum oven's regulation, evaporation becomes unpredictable. This often leads to warping, cracking, or the formation of large voids within the monolith, compromising its mechanical strength.
Electrochemical degradation
If the argon atmosphere is compromised, the material will oxidize immediately upon heating. An oxidized monolith may look structurally sound, but it will be electrically "dead," having lost the functional properties required for its intended application.
Making the Right Choice for Your Goal
When preparing densified MXene monoliths, your equipment setup dictates your results.
- If your primary focus is structural density: Ensure your vacuum settings are calibrated to slow the evaporation rate, allowing for uniform, conformal shrinkage.
- If your primary focus is electrochemical performance: Verify the integrity of your argon seal to completely exclude oxygen during the high-temperature dehydration phase.
Success lies in synchronizing these two factors: using vacuum to shape the material and argon to save its properties.
Summary Table:
| Process Requirement | Functional Role | Key Benefit |
|---|---|---|
| Vacuum Environment | Regulated solvent evaporation | Achieves conformal shrinkage without structural collapse |
| Argon Gas Protection | Inert atmospheric shielding | Prevents MXene oxidation and preserves conductivity |
| Controlled Heating | High-temperature dehydration | Facilitates densification while maintaining chemical properties |
| Uniform Pressure | Internal matrix stabilization | Eliminates voids, warping, and mechanical fractures |
Elevate Your Material Research with KINTEK Precision
Don't let oxidation or structural failure compromise your MXene research. KINTEK provides industry-leading, customizable Vacuum, Tube, and CVD systems designed to meet the rigorous demands of advanced material synthesis. Backed by expert R&D and precision manufacturing, our lab high-temperature furnaces ensure the perfect balance of controlled atmosphere and thermal stability for your unique densification needs.
Ready to achieve high-performance results? Contact our technical experts today to find the ideal furnace solution for your laboratory.
Visual Guide
References
- Boya Zhang, Ying Tao. Unlocking Unprecedented Gravimetric Capacitance in Thick Electrodes Through Conformal Densification of Robust MXene Hydrogels. DOI: 10.1002/adfm.202511313
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Vacuum Heat Treat Sintering and Brazing Furnace
- Dental Porcelain Zirconia Sintering Ceramic Vacuum Press Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What is the operating mechanism of a catalytic degreasing furnace? Master 17-4 PH Stainless Steel Debinding
- How does a bell-type plasma nitriding furnace enhance GGG60 ductile iron? Superior Surface Hardening Solutions
- Why must a vacuum system maintain 3.6 mbar for plasma nitriding? Master Precision Surface Hardening
- How does a vacuum annealing furnace contribute to microstructural recovery of ODS steel? Unlock Material Performance
- How does a vacuum furnace achieve energy efficiency? Superior Heat Containment and Optimized Cycles
- What are the primary process objectives of using a vacuum annealing furnace for treating HEA multilayer films?
- What are the key benefits of using a vacuum sintering furnace? Achieve Superior Material Purity and Process Control
- What are the steps involved in a typical vacuum brazing treatment? Master the Process for Strong, Clean Joints



















