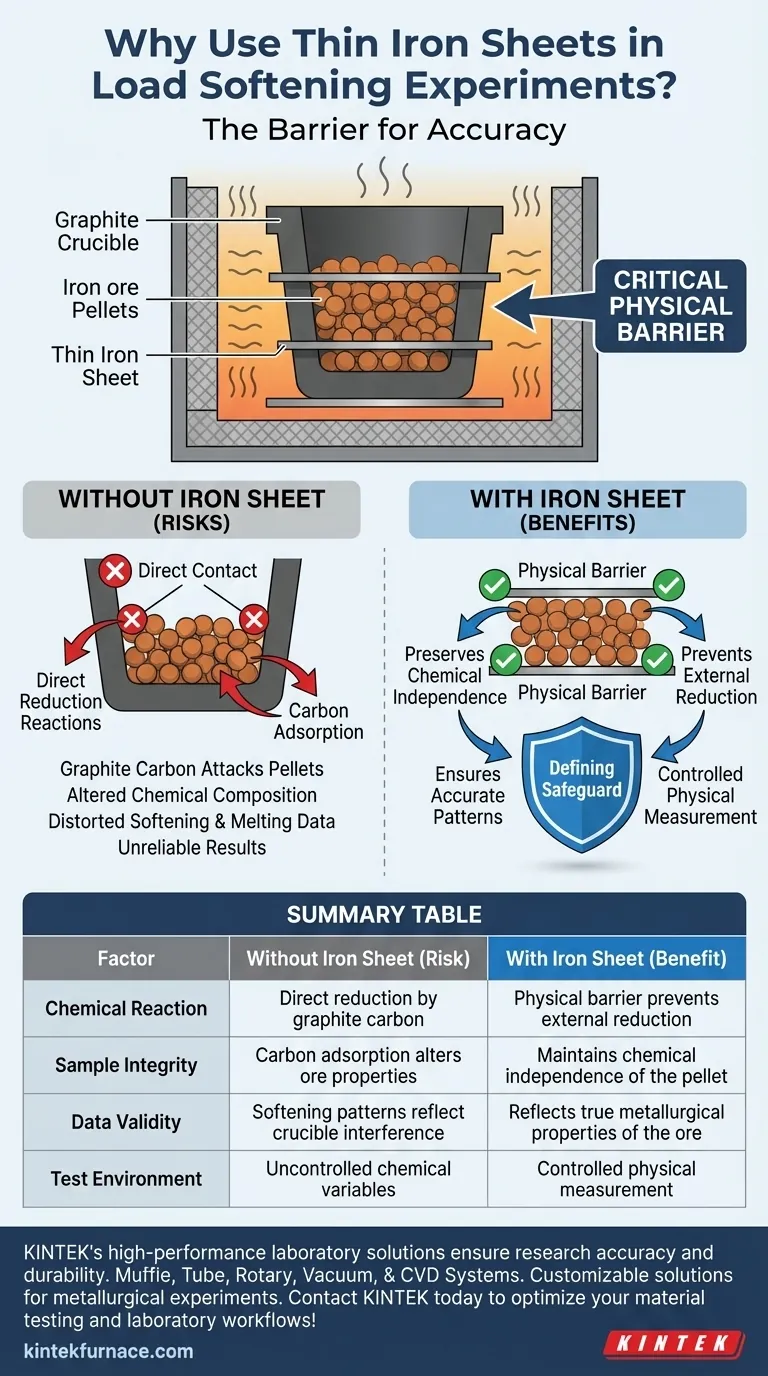
The primary function of thin iron sheets is to act as a critical physical barrier that isolates the iron ore pellets from the graphite crucible during high-temperature testing. By separating these materials, the sheets prevent unwanted chemical reactions that would otherwise compromise the integrity of the experiment.
By blocking direct contact between the iron oxides and the graphite, the sheets prevent external reduction reactions and carbon adsorption. This ensures that the resulting softening and melting data reflects the true properties of the ore, rather than the effects of crucible interference.
Preserving Chemical Independence
To understand why this barrier is necessary, you must look at the chemical volatility of the materials involved at high temperatures.
The Risk of Direct Reduction
Graphite is composed of carbon, while iron ore pellets consist largely of iron oxides.
At the elevated temperatures required for load softening experiments, carbon is a potent reducing agent.
Without a barrier, the graphite crucible would chemically attack the pellets, triggering direct reduction reactions that fundamentally alter the sample's composition during the test.
Preventing Carbon Adsorption
Beyond simple reduction, direct contact introduces the risk of carbon adsorption.
If carbon from the crucible migrates into the iron sample, it alters the metallurgical properties of the pellet.
The thin iron sheet effectively halts this migration, maintaining the chemical independence of the sample.
Ensuring Accurate Softening Patterns
The objective of the experiment is to measure when the ore softens and melts under load.
If the crucible material interacts with the sample, it changes the melting point and physical behavior of the ore.
The iron sheets ensure that the recorded softening and melting patterns are intrinsic to the ore, not artifacts of a reaction with the container.
The Risks of Material Interference
Failing to isolate the sample introduces variables that make data unreliable.
Compromised Experimental Data
When the crucible participates in the reaction, the environment inside the test chamber is no longer controlled solely by the experimenter's parameters.
The resulting data becomes a measurement of a reaction product rather than the original iron ore pellet.
Distorted Physical Behavior
Chemical interference often leads to premature or delayed softening compared to the material's natural behavior.
Using the iron sheets acts as a standardization measure, ensuring that interference from the crucible material is removed from the equation completely.
Ensuring Experimental Validity
To achieve reliable results in load softening experiments, you must prioritize sample isolation.
- If your primary focus is Chemical Purity: Ensure the iron sheets are intact and properly positioned to prevent any carbon migration from the crucible.
- If your primary focus is Data Accuracy: Recognize that the barrier is essential for capturing the true physical changes of the ore, free from external reduction effects.
The thin iron sheet is the defining safeguard that transforms a potential chemical reaction back into a controlled physical measurement.
Summary Table:
| Factor | Without Iron Sheet (Risk) | With Iron Sheet (Benefit) |
|---|---|---|
| Chemical Reaction | Direct reduction by graphite carbon | Physical barrier prevents external reduction |
| Sample Integrity | Carbon adsorption alters ore properties | Maintains chemical independence of the pellet |
| Data Validity | Softening patterns reflect crucible interference | Reflects true metallurgical properties of the ore |
| Test Environment | Uncontrolled chemical variables | Controlled physical measurement |
Precision in laboratory testing requires the right equipment and expert guidance. Backed by industry-leading R&D and manufacturing, KINTEK offers a comprehensive range of high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you need standard lab high-temp furnaces or fully customizable solutions for unique metallurgical experiments, our team ensures your research is supported by the highest standards of accuracy and durability. Contact KINTEK today to discover how our advanced thermal solutions can optimize your material testing and laboratory workflows!
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What effect does water-quench cooling equipment have on the microstructure of Invar 36? Expert Analysis
- Why do high-performance Bi-2223 superconducting materials require high-precision temperature control? | KINTEK Solution
- Why must high-purity nitrogen be used for biochar activation? Ensure Carbon Integrity and Pore Development
- How does a high-precision infrared temperature measurement system influence the sintering quality of Al2O3/TiC ceramics?
- What role does a laboratory drying oven play in catalyst supports? Ensure Structural Integrity & High Dispersion
- What role does a laboratory blast drying oven play in the preparation of Ti-doped Mn3O4? Optimize Your Precursor Quality
- How is a laboratory oven utilized during the impregnation stage of APC preparation? Optimize Biochar Activation
- Why is dual heat treatment required for SnO2 nanoparticles? Optimize Oxidation for Superior Performance



















