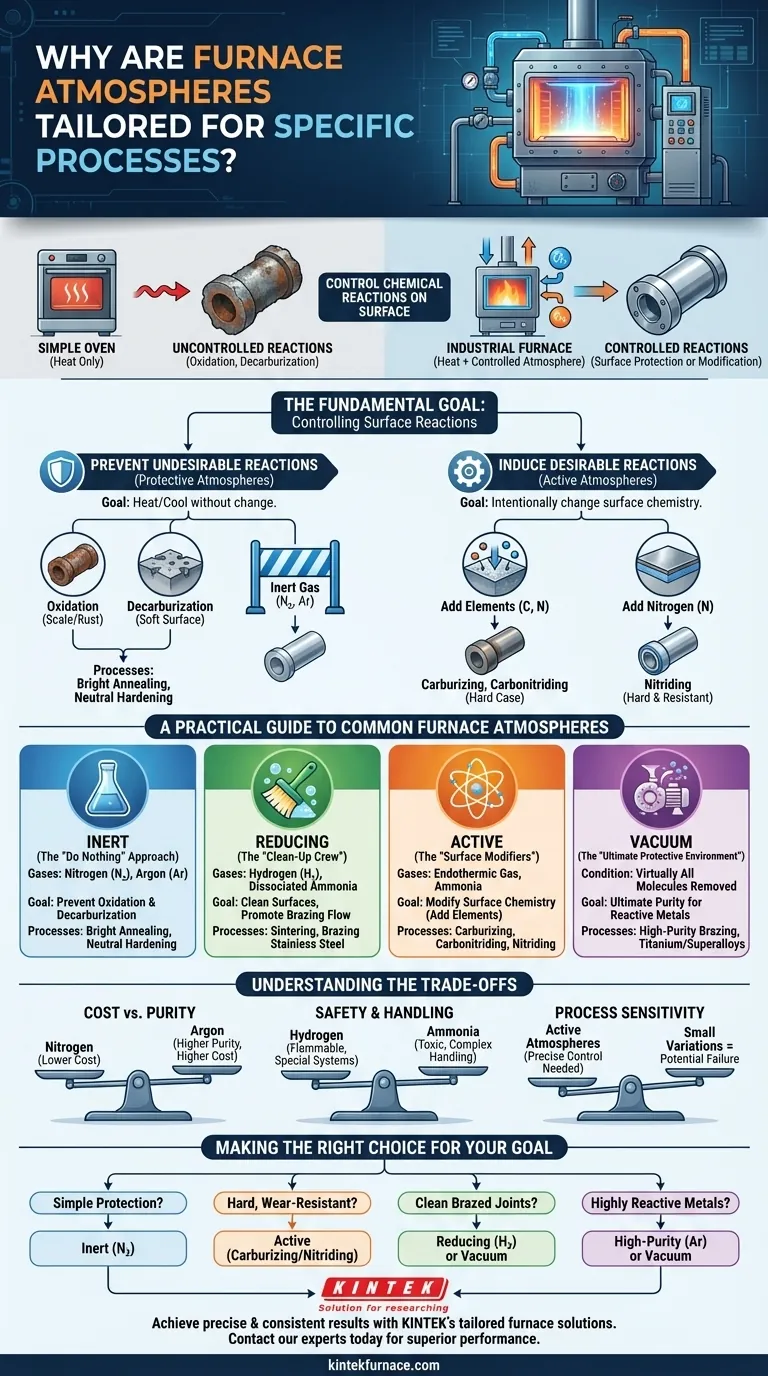
The short answer is that furnace atmospheres are precisely tailored to control chemical reactions on a material's surface at high temperatures. Without this control, processes like hardening, joining, or finishing would fail due to undesirable effects like oxidation or the loss of critical alloying elements from the material's surface. The atmosphere is not a passive environment; it is an active ingredient in the heat treatment process.
A simple oven just provides heat. An industrial furnace provides a combination of heat and a chemically controlled environment. This distinction is the core reason atmospheres are tailored: they are a critical tool used to either protect the material or to intentionally change its surface properties.

The Fundamental Goal: Controlling Surface Reactions
At the high temperatures found in industrial furnaces, materials become highly reactive. The air we breathe, composed of roughly 78% nitrogen and 21% oxygen, is incredibly corrosive to hot metals. The purpose of a controlled atmosphere is to replace the air with a specific gas mixture that dictates what happens on the part's surface.
Preventing Undesirable Reactions (Protective Atmospheres)
For many processes, the goal is simply to heat and cool a part without changing it. The primary enemies here are oxidation and decarburization.
Oxidation is the formation of scale or rust on the metal's surface, which can ruin surface finish and dimensional accuracy. An inert atmosphere displaces the oxygen to prevent this.
Decarburization is the loss of carbon from the surface of steel. Since carbon is the primary element providing hardness in steel, its loss results in a soft, weak surface layer that compromises the part's performance.
Processes like neutral hardening and bright annealing rely on protective atmospheres like nitrogen or argon to prevent both of these reactions, ensuring the part exits the furnace with the same surface chemistry it had when it went in.
Inducing Desirable Reactions (Active Atmospheres)
In other cases, the goal is to intentionally change the surface chemistry to enhance the material's properties. Here, the atmosphere becomes an active participant, donating elements to the part's surface.
Carburizing and carbonitriding use atmospheres rich in carbon and nitrogen. These elements diffuse into the surface of steel parts, creating a very hard, wear-resistant "case" over a softer, tougher core.
Nitriding uses a nitrogen-rich atmosphere, typically derived from ammonia, to create an extremely hard surface that also has excellent corrosion resistance.
A Practical Guide to Common Furnace Atmospheres
Different goals demand different gas mixtures. The choice depends entirely on the desired interaction—or lack thereof—between the gas and the material.
Inert Atmospheres: The "Do Nothing" Approach
The goal of an inert atmosphere is to be completely non-reactive. It serves as a simple, protective blanket.
- Common Gases: Nitrogen (N₂), Argon (Ar)
- Primary Use: Preventing oxidation and decarburization.
- Typical Processes: Bright annealing, neutral hardening of tool steels.
Reducing Atmospheres: The "Clean-Up Crew"
A reducing atmosphere not only prevents oxidation but can also actively remove existing light surface oxides.
- Common Gases: Hydrogen (H₂), Dissociated Ammonia (H₂ + N₂)
- Primary Use: Cleaning surfaces and promoting the flow of brazing alloys.
- Typical Processes: Sintering of metal powders, brazing of stainless steel.
Active Atmospheres: The "Surface Modifiers"
These atmospheres are designed to add specific elements to the material's surface to enhance its properties.
- Common Gases: Endothermic gas (for carburizing), Ammonia (for nitriding).
- Primary Use: Case hardening steels for improved wear and fatigue resistance.
- Typical Processes: Carbonitriding, gas nitriding.
Vacuum: The Ultimate Protective Environment
A vacuum isn't a gas, but it functions as the ultimate inert atmosphere by removing virtually all molecules that could react with the part.
- Primary Use: Processing highly sensitive or reactive materials where even trace impurities are unacceptable.
- Typical Processes: High-purity brazing, heat treating of titanium or superalloys.
Understanding the Trade-offs
Choosing an atmosphere isn't just about chemistry; it involves balancing cost, safety, and process requirements.
Cost vs. Purity
Nitrogen is the most common inert gas because it is relatively inexpensive. Argon provides superior protection for highly reactive metals but comes at a significantly higher cost.
Safety and Handling
Hydrogen is an excellent reducing agent, but it is highly flammable and requires specialized safety systems. Ammonia, used for nitriding, is toxic. These factors add complexity and cost to equipment and facility design.
Process Sensitivity
Active atmospheres like those for carburizing require extremely precise control. Small variations in gas composition, temperature, or time can drastically alter the final surface hardness and case depth, potentially ruining the entire batch of parts.
Making the Right Choice for Your Goal
Your choice of atmosphere is a direct function of what you need to accomplish at high temperatures.
- If your primary focus is simple protection against scale: An inert nitrogen atmosphere is the most common and cost-effective choice.
- If your primary focus is creating a hard, wear-resistant surface: An active atmosphere for carburizing or nitriding is necessary.
- If your primary focus is creating clean, strong brazed joints: A reducing hydrogen atmosphere or a vacuum is required to ensure proper alloy flow.
- If your primary focus is processing highly reactive or exotic metals: A high-purity argon atmosphere or a deep vacuum is essential to prevent contamination.
Ultimately, tailoring the furnace atmosphere is a fundamental requirement for achieving consistent and predictable results in modern metallurgy.
Summary Table:
| Atmosphere Type | Primary Goal | Common Gases | Typical Processes |
|---|---|---|---|
| Inert | Prevent Reactions (Protect) | Nitrogen (N₂), Argon (Ar) | Bright Annealing, Neutral Hardening |
| Reducing | Clean & Prevent Oxidation | Hydrogen (H₂), Dissociated Ammonia | Sintering, Brazing |
| Active | Modify Surface Chemistry | Endothermic Gas, Ammonia (NH₃) | Carburizing, Nitriding |
| Vacuum | Ultimate Purity & Protection | (Near-total gas removal) | High-Purity Brazing, Titanium Alloys |
Achieve precise and consistent results in your lab. The right furnace atmosphere is key to your process success. At KINTEK, we leverage our deep expertise in thermal processing and strong in-house manufacturing capabilities to provide advanced furnace solutions—including Muffle, Tube, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems—perfectly tailored to your unique requirements. Our strong deep customization capability ensures your furnace and its atmosphere control system are optimized for your specific materials and goals. Let's discuss your application – contact our experts today for a solution that delivers superior performance and reliability.
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- What are the development prospects of atmosphere box furnaces in the aerospace industry? Unlock Advanced Material Processing for Aerospace Innovation
- How do argon and nitrogen protect samples in vacuum furnaces? Optimize Your Thermal Process with the Right Gas
- What is inert gas technology used for in high-temperature atmosphere vacuum furnaces? Protect Materials and Speed Up Cooling
- Can box type high-temperature resistance furnaces control the atmosphere? Unlock Precision in Material Processing
- What are some specific applications of atmosphere furnaces in the ceramics industry? Enhance Purity and Performance



















