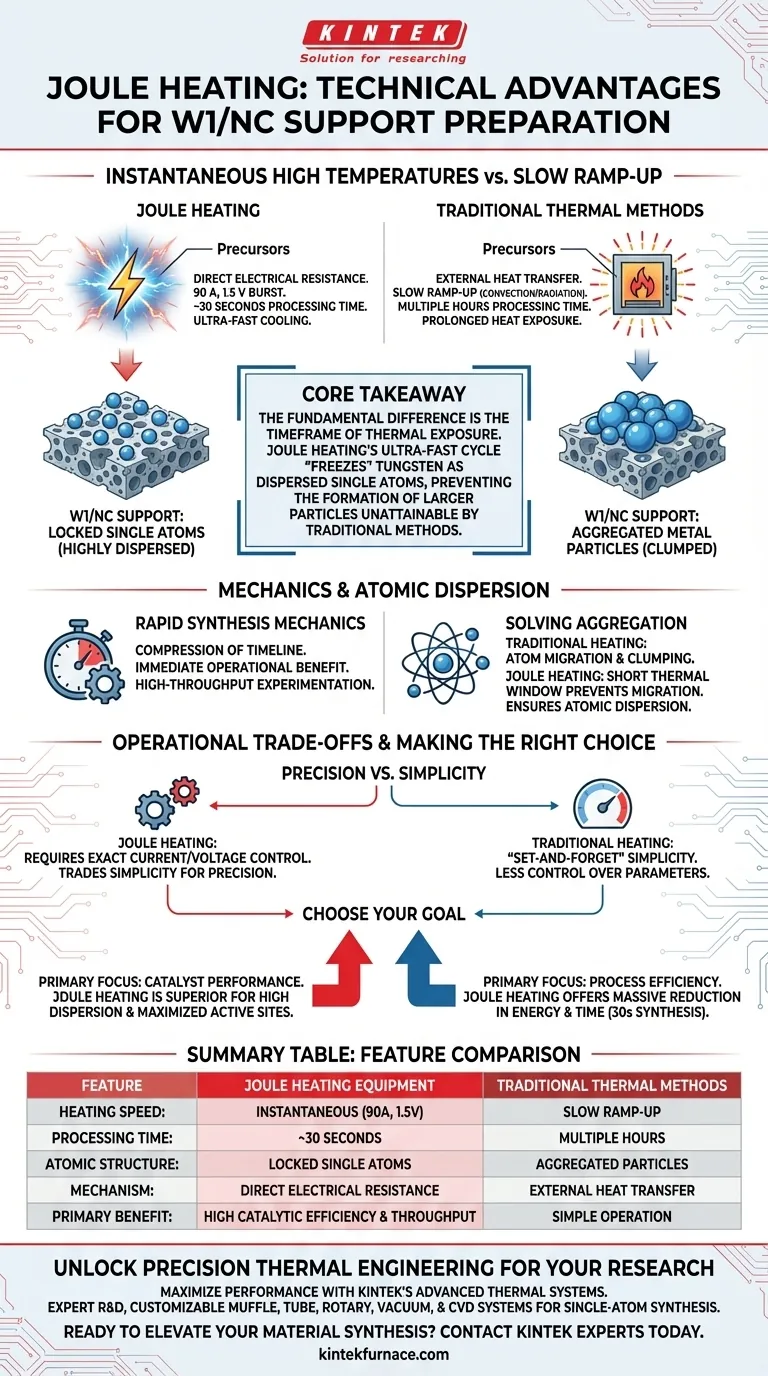
The primary technical advantage of Joule heating lies in its ability to generate instantaneous high temperatures through high-current electrical bursts, rather than external heat sources. By utilizing specific parameters (90 A, 1.5 V), this equipment facilitates the decomposition and transformation of precursors in a mere 30-second window, a speed unattainable by traditional thermal methods.
Core Takeaway: The fundamental difference is the timeframe of thermal exposure. Traditional heating keeps materials hot long enough for metal atoms to migrate and clump; Joule heating offers an ultra-fast heating and cooling cycle that effectively "freezes" tungsten as dispersed single atoms, preventing the formation of larger particles.

The Mechanics of Rapid Synthesis
Instantaneous Energy Delivery
Unlike traditional furnaces that rely on convection or radiation to slowly ramp up temperature, Joule heating uses direct electrical resistance.
By applying a high current of 90 A at a low voltage of 1.5 V, the equipment generates immediate, intense heat directly within the conductive material.
Drastic Reduction in Processing Time
The most immediate operational benefit is the compression of the synthesis timeline.
While traditional calcination may take hours, the Joule heating process completes the precursor transformation in just 30 seconds. This allows for high-throughput experimentation and production.
Achieving Atomic Dispersion
Solving the Aggregation Problem
A major failure point in preparing W1/NC (Tungsten/Nitrogen-doped Carbon) supports via traditional heating is the tendency for metal atoms to move.
Prolonged exposure to heat gives tungsten atoms the kinetic energy and time required to migrate across the support surface. This migration leads to aggregation, where atoms clump together to form large metal particles, reducing catalytic efficiency.
Locking in Single Atoms
Joule heating circumvents this issue through its ultra-fast cooling capabilities which immediately follow the heating burst.
Because the thermal window is so short, the tungsten (W) atoms do not have time to migrate and aggregate. This ensures the tungsten remains highly dispersed as single atoms on the porous carbon support.
Understanding the Operational Trade-offs
Precision vs. Simplicity
While Joule heating offers superior material quality for single-atom catalysts, it requires precise control over electrical parameters.
Traditional heating is often more "set-and-forget," whereas Joule heating demands exact management of current (Amperage) and voltage to prevent overheating or material destruction. You are trading the simplicity of a furnace for the precision of an electrical circuit.
Making the Right Choice for Your Goal
To determine if switching to Joule heating is necessary for your W1/NC preparation, consider your specific targets:
- If your primary focus is Catalyst Performance: Joule heating is superior because it ensures high dispersion of single atoms, maximizing the active sites available for reaction.
- If your primary focus is Process Efficiency: The 30-second synthesis time offers a massive reduction in energy consumption and time compared to traditional methods.
Summary: For the specific preparation of W1/NC supports, Joule heating is not just faster; it is the technical solution required to physically prevent metal particle aggregation.
Summary Table:
| Feature | Joule Heating Equipment | Traditional Thermal Methods |
|---|---|---|
| Heating Speed | Instantaneous (90 A, 1.5 V burst) | Slow Ramp-up (Convection/Radiation) |
| Processing Time | ~30 Seconds | Multiple Hours |
| Atomic Structure | Locked Single Atoms (Highly Dispersed) | Aggregated Metal Particles (Clumped) |
| Mechanism | Direct Electrical Resistance | External Heat Transfer |
| Primary Benefit | High Catalytic Efficiency & Throughput | Simple, Set-and-Forget Operation |
Unlock Precision Thermal Engineering for Your Research
Maximize your catalytic performance with the speed and precision of advanced thermal systems. Backed by expert R&D and manufacturing, KINTEK offers state-of-the-art Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as specialized high-temperature lab furnaces—all fully customizable to meet the rigorous demands of single-atom catalyst synthesis and material science.
Whether you need to replicate rapid thermal cycles or maintain exact atmospheric control, our team provides the technical expertise to enhance your lab's efficiency.
Ready to elevate your material synthesis? Contact KINTEK experts today to find the perfect customizable solution for your unique research needs.
Visual Guide
References
- Wensheng Jiao, Yunhu Han. All-round enhancement induced by oxophilic single Ru and W atoms for alkaline hydrogen oxidation of tiny Pt nanoparticles. DOI: 10.1038/s41467-025-56240-y
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What roles does a laboratory constant-temperature drying oven play in evaluating eggshell adsorbents? Key Insights
- What is the primary role of an industrial-grade oven in the preparation of chitosan-modified soil samples?
- Why is industrial-grade nitrogen flow introduced during the biochar pyrolysis process? Ensure Safety and Quality
- Why is HR-TEM used after high-temperature heat treatment? Visualize structural evolution and material integrity.
- What are the key differences between batch and continuous processing furnaces? Optimize Your Thermal Processing Strategy
- What is the technical purpose of drying NaNbO3:Pr3+ precursors at 60 °C? Optimize Your Powder Synthesis
- What are the process advantages of using template synthesis for the preparation of zinc selenide (ZnSe)?
- What are the objectives of melt stirring and insulation treatment during the Al-5Er-Ti master alloy preparation process?



















