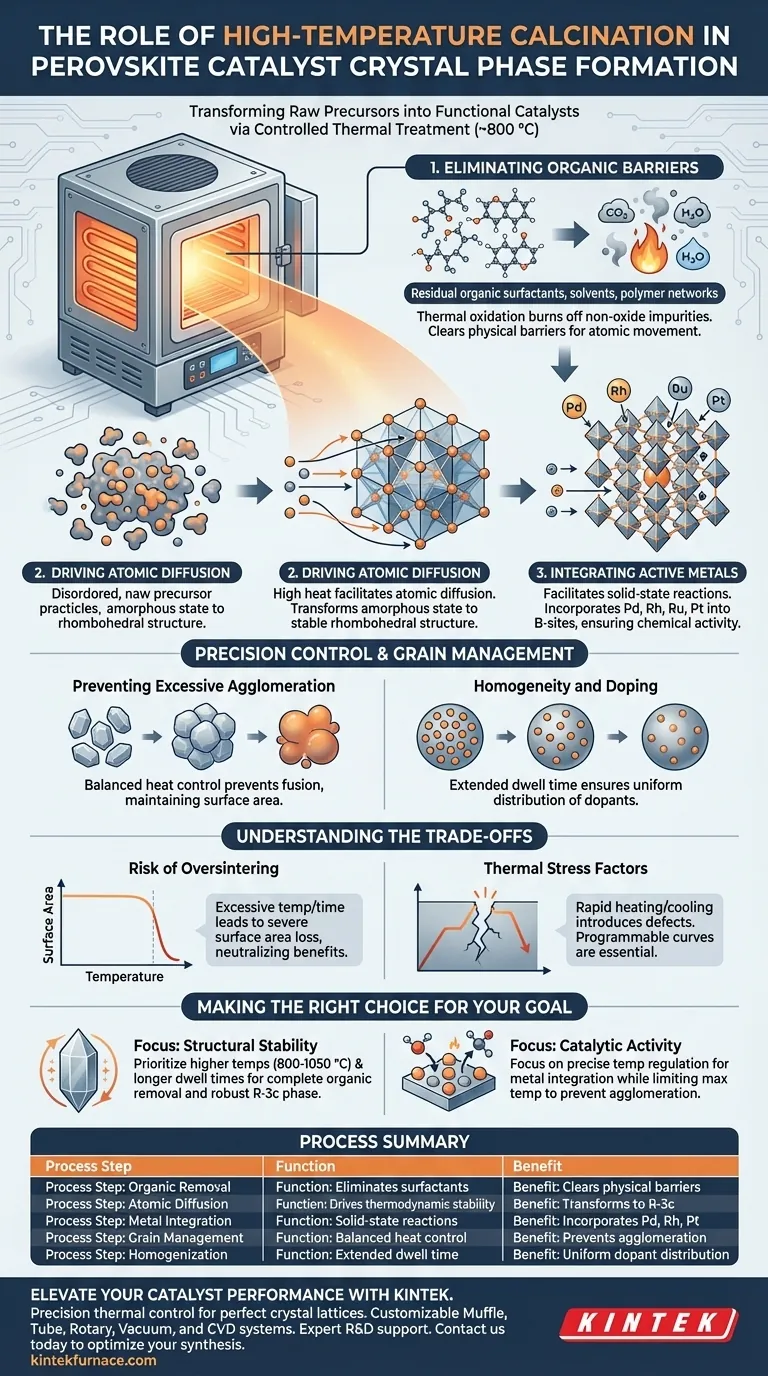
A high-temperature calcination furnace acts as the definitive processing tool that transforms raw precursor powders into functional perovskite catalysts. By maintaining a controlled environment around 800 °C, the furnace drives the removal of organic residues and facilitates the atomic diffusion required to stabilize the material. This process is essential for converting disordered mixtures into a highly ordered rhombohedral crystal structure.
The furnace does not merely dry the material; it engineers the lattice. It provides the activation energy necessary to integrate noble metal ions into specific atomic sites, ensuring the catalyst is not only stable but chemically active.

The Mechanics of Crystal Phase Formation
Eliminating Organic Barriers
Before a stable crystal phase can form, the precursor material must be purged of non-oxide impurities.
The calcination furnace subjects the powder to extended thermal treatment in an air environment. This thermal oxidation completely burns off residual organic surfactants, solvents, and polymer networks left over from the initial synthesis or combustion steps. Removing these physical barriers is a prerequisite for the atomic movement described below.
Driving Atomic Diffusion
Once impurities are removed, the material requires significant energy to reorganize its internal structure.
The high heat facilitates atomic diffusion, allowing atoms to migrate and settle into their most thermodynamically stable positions. For many perovskite catalysts, this results in a transformation from an amorphous or disordered state into a specific rhombohedral structure (R-3c space group). This structural order is what defines the material's physical properties.
Integrating Active Metals
For a perovskite to function as a catalyst, specific active metals must be incorporated into the crystal lattice.
The furnace facilitates the solid-state reactions necessary to integrate noble metal ions—such as Pd, Rh, Ru, or Pt—directly into the B-sites of the perovskite lattice. Without the sustained high temperature provided by the furnace, these metals might remain on the surface as separate phases rather than becoming an intrinsic part of the active crystal structure.
Precision Control and Grain Management
Preventing Excessive Agglomeration
While high heat promotes crystallization, uncontrolled heat can fuse particles together effectively destroying the surface area.
A properly regulated calcination furnace offers precise temperature control to balance crystal growth against particle fusion. This precision ensures the formation of well-crystallized phases while preventing excessive agglomeration of grains, maintaining the surface area required for catalytic reactions.
Homogeneity and Doping
The duration of the calcination process helps ensure the material is uniform throughout.
Long-duration heat treatment allows for the uniform distribution of dopants, such as nickel species, either within the lattice or across particle surfaces. This homogeneity is critical for ensuring that the catalyst performs consistently across its entire volume.
Understanding the Trade-offs
The Risk of Oversintering
While high temperatures are necessary for phase formation, there is a fine line between calcination and sintering.
If the temperature exceeds the optimal range (e.g., significantly above 800 °C for specific formulations) or if the dwell time is too long, the material may suffer from severe surface area loss. This reduces the number of exposed active sites, potentially neutralizing the benefits of the improved crystal structure.
Thermal Stress Factors
Rapid heating or cooling curves can introduce defects rather than removing them.
Programmable furnaces are often required to manage the heating rate. A curve that is too aggressive can trap organic residues inside the lattice before they oxidize or cause thermal shock that fractures the newly formed crystals.
Making the Right Choice for Your Goal
When configuring your calcination process, your specific objectives should dictate your parameters:
- If your primary focus is Structural Stability: Prioritize higher temperatures (e.g., 800 °C to 1050 °C) and longer dwell times to ensure complete organic removal and the formation of the robust R-3c phase.
- If your primary focus is Catalytic Activity: Focus on precise temperature regulation to integrate noble metals into B-sites while strictly limiting the maximum temperature to prevent surface area loss via agglomeration.
Ultimately, the calcination furnace is the gatekeeper that determines whether your material becomes a generic powder or a high-performance catalyst.
Summary Table:
| Process Step | Function in Crystal Phase Formation | Key Benefit |
|---|---|---|
| Organic Removal | Eliminates surfactants and polymer networks | Clears physical barriers for atomic movement |
| Atomic Diffusion | Drives atoms into thermodynamic stability | Transforms amorphous state to rhombohedral (R-3c) |
| Metal Integration | Facilitates solid-state reactions | Incorporates Pd, Rh, and Pt into the B-site lattice |
| Grain Management | Balanced heat control | Prevents excessive agglomeration & surface area loss |
| Homogenization | Extended dwell time | Ensures uniform dopant distribution (e.g., Nickel) |
Elevate Your Catalyst Performance with KINTEK
Precision is the difference between a generic powder and a high-performance perovskite catalyst. At KINTEK, we understand that the perfect crystal lattice requires exact thermal control. Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to master the delicate balance of high-temperature calcination.
Why choose KINTEK for your lab?
- Customizable Systems: Tailored thermal curves for specific R-3c phase stabilization.
- Precise Temperature Regulation: Prevent oversintering and preserve active surface sites.
- Expert Support: Specialized high-temp furnaces built for unique R&D and manufacturing needs.
Ready to optimize your material synthesis? Contact us today to find the perfect customizable furnace for your research requirements.
Visual Guide
References
- Pradeep Kumar Yadav, Sudhanshu Sharma. Effect of Partial Noble Metal (M = Pd, Rh, Ru, Pt) Substitution in La1−xSrxCo1−yMyO3 Perovskite-Derived Catalysts for Dry Reforming of Methane. DOI: 10.3390/hydrogen6030049
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What role do auxiliary equipment like fans and sprayers play in a box furnace? Enhance Precision and Efficiency in Heat Treatment
- How does the programmable temperature control of a high-precision box resistance furnace influence the properties of pyrolyzed composite materials?
- What are the key features of modern electrical muffle furnaces? Achieve Precise, Pure, and Efficient High-Temp Processing
- What are the alternative names for a muffle furnace? Discover Key Terms and Design Insights
- What role does a muffle furnace play in the single-step pyrolysis of magnetic chitosan carbon? Streamline Synthesis
- What is the key role of a muffle furnace in the pretreatment of boron sludge and szaibelyite? Unlock Higher Process Efficiency
- What role does a high-temperature muffle furnace play in STFO synthesis? Achieve Pure Perovskite Results
- What is the primary purpose of using a muffle furnace for MAL calcination? Unlock the Structure Memory Effect



















