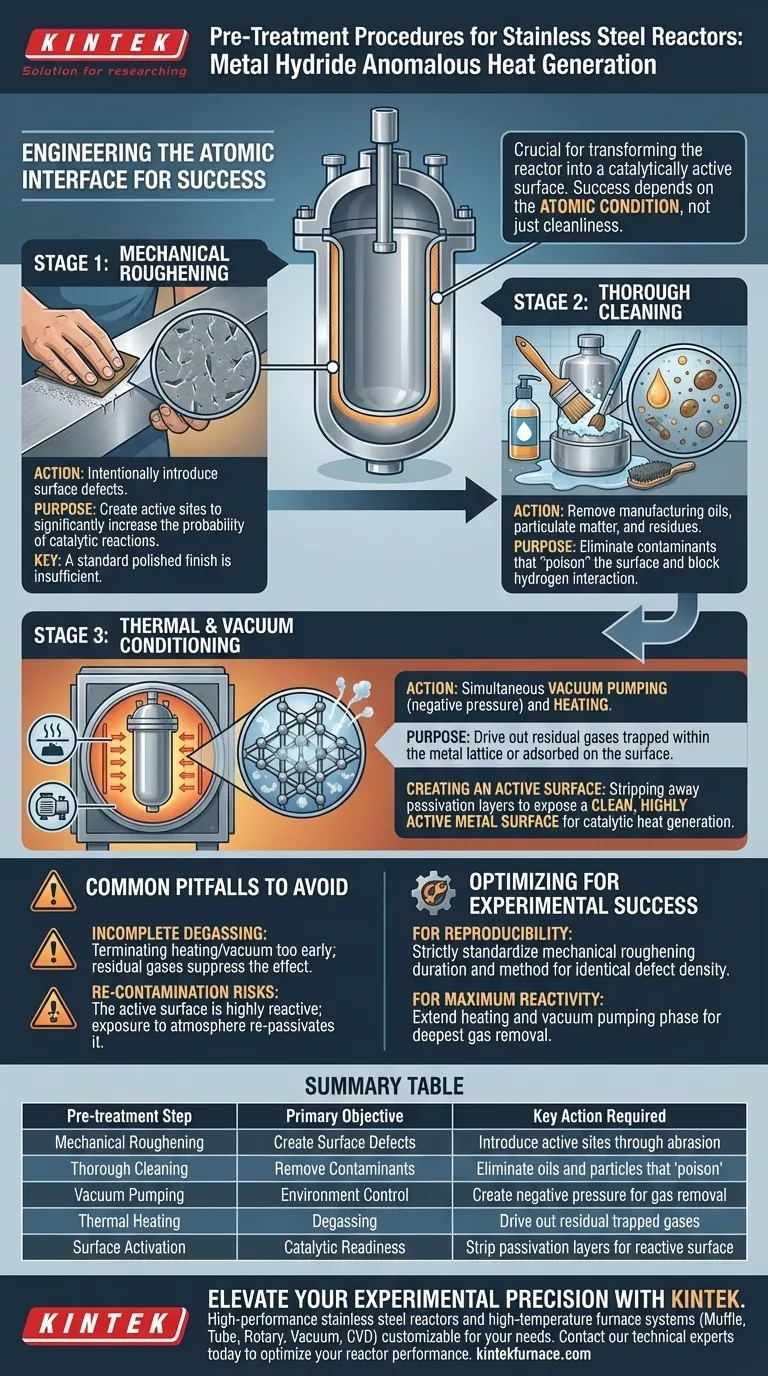
To properly prepare a Stainless Steel Reactor for metal hydride experiments, you must perform a rigorous sequence of physical roughening, thorough cleaning, and thermal degassing under vacuum. These specific pre-treatment steps are mandatory to transform the reactor walls into a catalytically active surface capable of supporting anomalous heat generation.
Success in anomalous heat generation is determined by the atomic condition of the reactor interface. Pre-treatment is not merely about cleanliness; it is an engineering process designed to create specific surface defects and eliminate contaminants that would otherwise inhibit the hydrogen-metal reaction.

Engineering the Reactor Surface
To enable the necessary interactions between hydrogen and the metal, you must modify the physical characteristics of the steel.
Mechanical Roughening
A standard, polished finish is insufficient for these experiments. You must subject the reactor to mechanical roughening.
This process is designed to intentionally introduce surface defects. These defects serve as active sites where the probability of the required catalytic reactions is significantly increased.
Thorough Cleaning
Once the surface structure is modified, the reactor requires thorough cleaning.
This step is critical to remove any manufacturing oils, particulate matter, or residues introduced during the roughening process. Any remaining contaminants can "poison" the surface, blocking hydrogen from interacting with the metal lattice.
Thermal and Vacuum Conditioning
Physical preparation must be followed by chemical and thermal conditioning to ensure the metal is chemically active.
Vacuum Degassing
The reactor must undergo vacuum pumping to create a negative pressure environment.
Simultaneously, the reactor must be subjected to heating. This combination of heat and vacuum is the only reliable method to drive out residual gases trapped within the metal or adsorbed onto the surface.
Creating an Active Surface
The ultimate goal of this heating and pumping cycle is to strip away passivation layers and impurities.
This leaves you with a clean, highly active metal surface. This state is essential for facilitating the catalytic reactions required for heat generation between the hydrogen gas and the metal surface.
Common Pitfalls to Avoid
While the steps are straightforward, the margin for error in these experiments is narrow.
Incomplete Degassing
A common failure point is terminating the heating and vacuum phase too early.
If residual internal gases remain, they can outgas during the experiment. This introduces variables that can suppress the anomalous heat effect or lead to false data.
Re-contamination Risks
The "active surface" created by this process is highly reactive and unstable.
Exposure to standard atmosphere or improper handling after treatment can instantly re-passivate or contaminate the surface. This renders the previous cleaning steps useless.
Optimizing for Experimental Success
When planning your pre-treatment protocol, align your procedures with your specific experimental goals.
- If your primary focus is Reproducibility: Strictly standardize the method and duration of your mechanical roughening to ensure the density of surface defects is identical across different reactor builds.
- If your primary focus is Maximum Reactivity: Extend the duration of the heating and vacuum pumping phase to ensure the deepest possible removal of residual internal gases.
Treat the reactor surface not as a passive container, but as an active participant in the reaction.
Summary Table:
| Pre-treatment Step | Primary Objective | Key Action Required |
|---|---|---|
| Mechanical Roughening | Create Surface Defects | Introduce active sites for catalytic reactions through abrasion |
| Thorough Cleaning | Remove Contaminants | Eliminate oils and particles that 'poison' the metal surface |
| Vacuum Pumping | Environment Control | Create negative pressure to facilitate gas removal |
| Thermal Heating | Degassing | Drive out residual trapped gases from the metal lattice |
| Surface Activation | Catalytic Readiness | Strip passivation layers to expose a highly reactive metal surface |
Elevate Your Experimental Precision with KINTEK
Don’t let surface contamination or inadequate degassing compromise your results. KINTEK provides high-performance stainless steel reactors and high-temperature furnace systems engineered for the most demanding research environments.
Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your specific pre-treatment and thermal processing needs. Whether you are aiming for maximum reactivity or perfect reproducibility, our equipment delivers the uniform heating and vacuum stability your metal hydride experiments require.
Ready to optimize your reactor performance? Contact our technical experts today to discuss your custom laboratory solution.
Visual Guide
References
- Tadahiko Mizuno, Jed Rothwell. Anomalous Heat Reaction from Hydrogen and Metals. DOI: 10.70923/001c.134027
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What are the typical applications for drying ovens? Essential Uses in Labs and Industry
- How does a constant temperature drying oven contribute to MgTiO3-CaTiO3 ceramic slurry? Optimize Your Precursor Quality
- What is the role of a precision heating system in HEA synthesis? Achieve Atomic Uniformity at 220 °C
- How is the pore structure of EN-LCNF characterized? Advanced BET and DFT Analysis of Carbon Nanosheets
- How is the problem of surface oxidation and decarburization addressed in conventional heat treatment? Learn the Machining Allowance Method
- What is the function of industrial furnaces in 7075 aluminum solution treatment? Master Material Strength
- What is the primary purpose of high-temperature pyrolysis? Unlock Superior PFAS Removal with Enhanced Hydrophobicity
- What role does an RTA system play in Zirconia preparation? Master Phase Transformation for Advanced Deposition



















