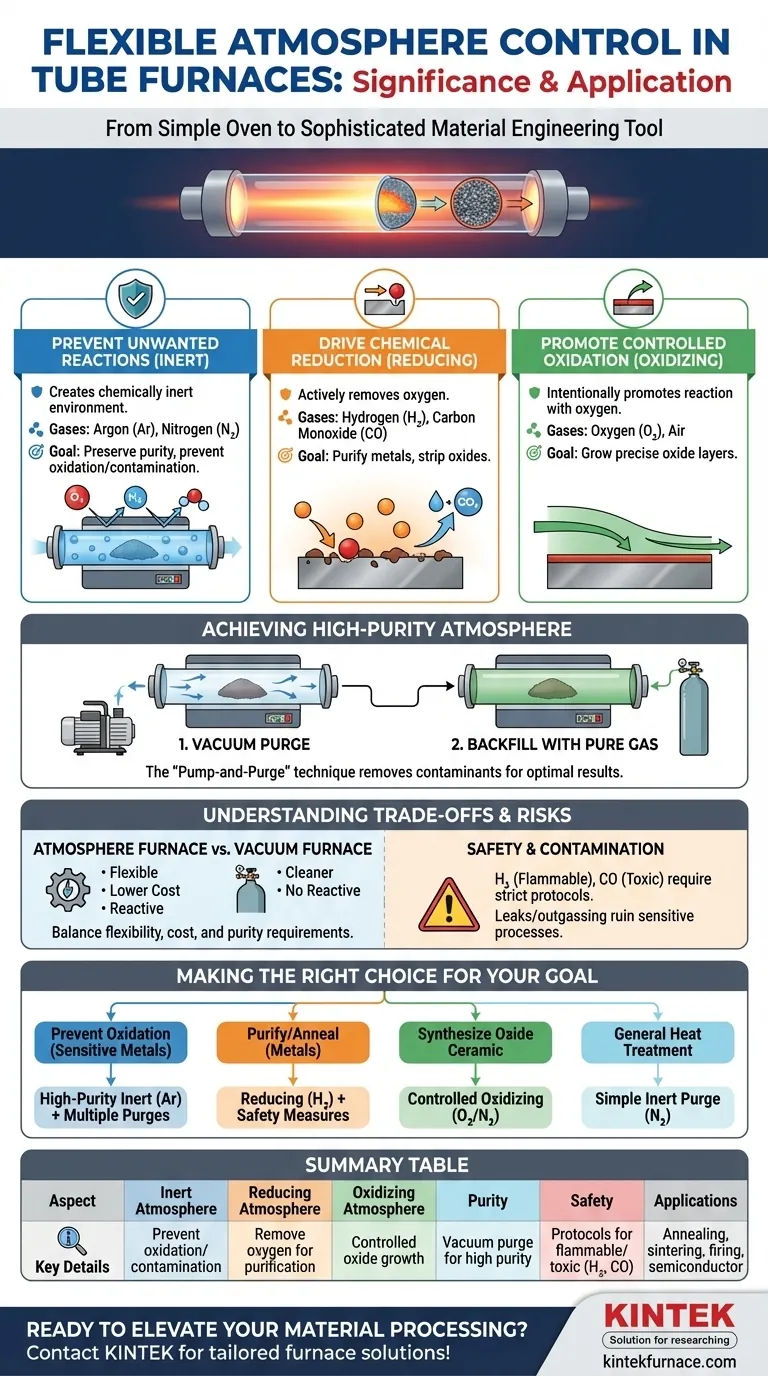
At its core, the significance of flexible atmosphere control in a tube furnace is its ability to create a precisely defined chemical environment for a material during heat treatment. This allows you to go beyond simple heating and actively direct chemical reactions, preventing unwanted changes like oxidation or intentionally causing desired ones, such as reduction or specific oxide growth, to fundamentally alter and improve a material's final properties.
The key takeaway is that atmosphere control transforms a furnace from a simple oven into a sophisticated processing tool. It's not just about protecting your sample from air; it's about using a specific gas environment to actively engineer the material's structure and performance at a microscopic level.

The Role of Atmosphere in Material Transformation
The gas surrounding your sample during heat treatment is not a passive bystander; it is an active chemical reagent. Controlling this atmosphere is fundamental to achieving reproducible and targeted results in materials science and manufacturing.
Preventing Unwanted Reactions: The Inert Atmosphere
The most common goal of atmosphere control is to create a chemically inert environment. This prevents the sample from reacting with oxygen and water vapor present in ambient air, which can cause unwanted oxidation and contamination, especially at high temperatures.
Gases like Argon (Ar) and Nitrogen (N₂) are used for this purpose. They displace the reactive air, preserving the material's purity and intended composition during processes like annealing or sintering sensitive metals.
Driving Chemical Reduction: The Reducing Atmosphere
A reducing atmosphere is one that actively removes oxygen. This is critical for processes where oxides are undesirable or need to be stripped away from a material's surface.
Gases like Hydrogen (H₂) or Carbon Monoxide (CO) are introduced to react with and remove oxygen. This is essential for purifying certain metals or preparing materials that must be free of oxides to function correctly.
Promoting Controlled Oxidation: The Oxidizing Atmosphere
Conversely, sometimes you want to intentionally promote a reaction with oxygen. An oxidizing atmosphere allows for the controlled growth of an oxide layer on a material.
Introducing a specific concentration of Oxygen (O₂) or clean air is common in the firing of certain ceramics or in semiconductor manufacturing, where precise oxide layers are required for device functionality.
Achieving a High-Purity Atmosphere
Simply flowing a gas into the furnace tube is often insufficient. To ensure the atmosphere is pure and free from contaminants, a specific procedure is required for optimal results.
The Vacuum Purge Technique
The most effective method is to first use a vacuum pump to evacuate the air from the sealed furnace tube. This removes the vast majority of residual oxygen, moisture, and other contaminants.
Once a vacuum is established, the chamber is backfilled with the high-purity process gas (e.g., Argon). For highly sensitive experiments, this "pump-and-purge" cycle can be repeated several times to achieve an exceptionally pure atmosphere inside the tube.
Understanding the Trade-offs and Considerations
While powerful, atmosphere control is not without its complexities. Making the right choice involves understanding the trade-offs between different equipment and the safety requirements of various gases.
Atmosphere Furnace vs. Vacuum Furnace
An atmosphere furnace offers great flexibility and is significantly lower in cost than a high-vacuum furnace. It excels at creating specific reactive or inert gas environments.
However, a vacuum furnace provides a "cleaner" environment by removing nearly all gas molecules. It is superior for applications where even trace amounts of gas are unacceptable, but it cannot be used to create reactive atmospheres for processes like controlled oxidation.
Safety with Reactive Gases
Using reactive gases introduces significant safety considerations. Gases like Hydrogen are highly flammable, while Carbon Monoxide is extremely toxic.
Proper ventilation, gas leak detectors, and established safety protocols are non-negotiable when working with these gases to prevent accidents.
Purity and Contamination Risks
The final purity of your furnace atmosphere depends on the purity of your source gas and the cleanliness of your system. Leaks in fittings or outgassing from a dirty furnace tube can introduce contaminants that ruin a sensitive process.
Making the Right Choice for Your Goal
Your process objective dictates the type of atmosphere you need. By matching the gas environment to your material goal, you can ensure successful and repeatable outcomes.
- If your primary focus is preventing oxidation of a sensitive metal: Use a high-purity inert gas like Argon and perform multiple vacuum purge cycles to ensure maximum cleanliness.
- If your primary focus is synthesizing a specific oxide ceramic: Use a controlled flow of an oxidizing gas, such as a precise mixture of oxygen and nitrogen, to drive the desired reaction.
- If your primary focus is purifying a material or annealing a metal: Use a reducing atmosphere containing hydrogen, ensuring all safety measures for flammable gases are strictly followed.
- If your primary focus is general heat treatment with moderate protection: A simple purge with an inert gas like Nitrogen may be sufficient and more cost-effective than using more expensive Argon.
Mastering atmosphere control gives you direct command over the chemical destiny of your material, unlocking new possibilities for innovation and quality.
Summary Table:
| Aspect | Key Details |
|---|---|
| Inert Atmosphere | Uses Argon or Nitrogen to prevent oxidation and contamination. |
| Reducing Atmosphere | Employs Hydrogen or Carbon Monoxide to remove oxygen for purification. |
| Oxidizing Atmosphere | Introduces Oxygen or air for controlled oxide layer growth. |
| Atmosphere Purity | Achieved via vacuum purge techniques for high-purity environments. |
| Safety Considerations | Requires protocols for flammable/toxic gases like Hydrogen and CO. |
| Applications | Includes annealing, sintering, ceramic firing, and semiconductor processes. |
Ready to elevate your material processing with advanced atmosphere control? At KINTEK, we specialize in high-temperature furnace solutions tailored to your unique needs. Leveraging exceptional R&D and in-house manufacturing, our product line—including Tube Furnaces, Muffle Furnaces, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems—is designed for precision and reliability. With strong deep customization capabilities, we ensure your furnace meets exact experimental requirements, from inert gas environments to reactive processes. Don't let contamination or inconsistent results hold you back—contact us today to discuss how our expertise can optimize your lab's performance and drive innovation in your materials research!
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
People Also Ask
- How do argon and nitrogen protect samples in vacuum furnaces? Optimize Your Thermal Process with the Right Gas
- Can box type high-temperature resistance furnaces control the atmosphere? Unlock Precision in Material Processing
- How does the pressure range change under vacuum conditions in an atmosphere box furnace? Explore Key Shifts for Material Processing
- What is inert gas technology used for in high-temperature atmosphere vacuum furnaces? Protect Materials and Speed Up Cooling
- What are some specific applications of atmosphere furnaces in the ceramics industry? Enhance Purity and Performance



















