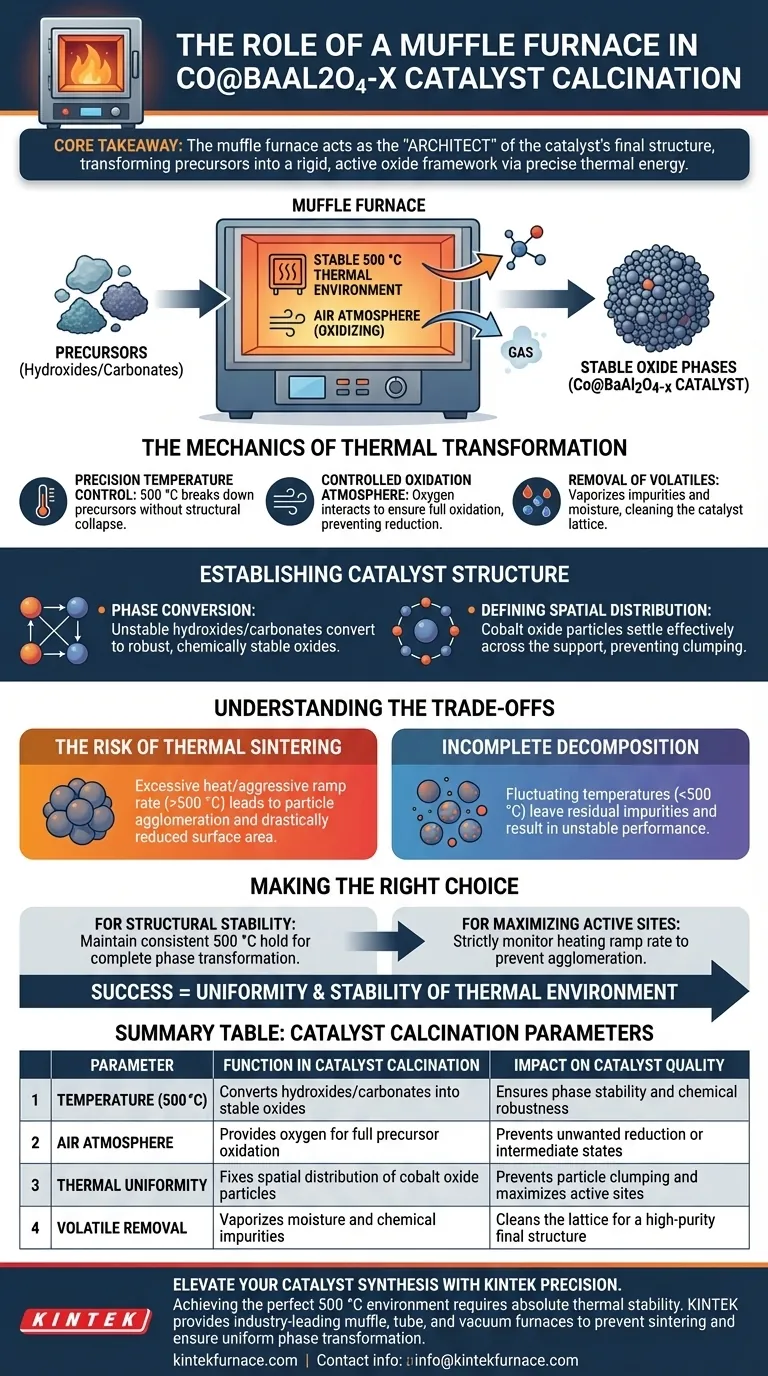
The primary role of a muffle furnace in this process is to provide a stable, controlled thermal environment at 500 °C under an air atmosphere. This specific heat treatment converts the unstable hydroxide or carbonate precursors—resulting from co-precipitation—into thermally stable oxide phases. Beyond simple drying, this step drives the chemical reactions necessary to remove volatile impurities and fixes the spatial distribution of cobalt oxide particles upon the barium-aluminum oxide support.
Core Takeaway: The muffle furnace acts as the "architect" of the catalyst's final structure. By applying precise thermal energy in an oxidizing atmosphere, it transforms raw chemical precursors into a rigid, active oxide framework with the correct spatial arrangement required for catalytic performance.

The Mechanics of Thermal Transformation
Precision Temperature Control
The muffle furnace maintains a rigorous temperature of 500 °C. This specific thermal energy level is calibrated to be high enough to break down precursors but controlled enough to prevent the collapse of the material's structure.
Controlled Oxidation Atmosphere
The process occurs specifically under an "air atmosphere." The muffle furnace allows oxygen to interact with the sample, ensuring the precursors oxidize fully rather than reducing or remaining in an intermediate state.
Removal of Volatiles
During co-precipitation, various impurities and moisture become trapped in the solid. The furnace provides the thermal drive required to vaporize these volatile components, effectively cleaning the catalyst lattice before the final structure sets.
Establishing Catalyst Structure
Phase Conversion
The raw material enters the furnace as hydroxides or carbonates, which are chemically unstable for this application. The thermal treatment drives a solid-phase reaction that converts these compounds into robust oxides (Co@BaAl2O4-x), which are chemically stable and ready for operation.
Defining Spatial Distribution
This is arguably the most critical function. As the precursors decompose, the cobalt species settle onto the support. The furnace's steady heat ensures that the cobalt oxide particles are distributed effectively across the barium-aluminum oxide support, rather than clumping randomly.
Understanding the Trade-offs
The Risk of Thermal Sintering
While heat is necessary for formation, excessive heat or lack of control can lead to sintering. If the temperature significantly exceeds the optimal 500 °C or the heating rate is too aggressive, the particles may agglomerate, drastically reducing the surface area and catalytic activity.
Incomplete Decomposition
Conversely, if the temperature fluctuates below the target or the duration is insufficient, the hydroxide or carbonate precursors may not fully decompose. This leaves residual impurities in the lattice, resulting in an unstable catalyst with unpredictable performance.
Making the Right Choice for Your Goal
To optimize the synthesis of Co@BaAl2O4-x catalysts, consider the following parameters:
- If your primary focus is Structural Stability: Ensure the furnace maintains a consistent hold at 500 °C to guarantee the complete phase transformation of hydroxides into robust oxides.
- If your primary focus is Maximizing Active Sites: strictly monitor the heating ramp rate to prevent rapid agglomeration, ensuring the cobalt oxide particles remain well-dispersed on the support.
Success depends not just on reaching 500 °C, but on the uniformity and stability of the thermal environment provided by the furnace.
Summary Table:
| Parameter | Function in Catalyst Calcination | Impact on Catalyst Quality |
|---|---|---|
| Temperature (500 °C) | Converts hydroxides/carbonates into stable oxides | Ensures phase stability and chemical robustness |
| Air Atmosphere | Provides oxygen for full precursor oxidation | Prevents unwanted reduction or intermediate states |
| Thermal Uniformity | Fixes spatial distribution of cobalt oxide particles | Prevents particle clumping and maximizes active sites |
| Volatile Removal | Vaporizes moisture and chemical impurities | Cleans the lattice for a high-purity final structure |
Elevate Your Catalyst Synthesis with KINTEK Precision
Achieving the perfect 500 °C environment for Co@BaAl2O4-x catalysts requires more than just heat—it requires absolute thermal stability. KINTEK provides industry-leading muffle furnaces, tube furnaces, and vacuum systems designed to prevent sintering and ensure uniform phase transformation.
Backed by expert R&D and manufacturing, our laboratory high-temp furnaces are fully customizable to meet your unique chemical synthesis needs. Don't compromise on your catalytic activity—contact our experts today to find the ideal thermal solution for your lab.
Visual Guide
References
- Pei Xiong, Molly Meng‐Jung Li. Efficient Low‐temperature Ammonia Cracking Enabled by Strained Heterostructure Interfaces on Ru‐free Catalyst. DOI: 10.1002/adma.202502034
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What role does a Muffle Furnace play in Bouligand ceramic aerogel production? Essential Ceramization & Solidification
- Why is a high-temperature laboratory furnace used for the recalcination of deactivated LDH catalysts? Restore Performance
- What role does a high-temperature laboratory oven play in catalyst activation? Boost Surface Area and Performance
- What are some general-purpose uses of muffle furnaces? Essential for Clean, High-Temperature Processing
- What role does a muffle furnace play in the synthesis of carbon nitride? Master Thermal Polycondensation Control
- What is the purpose of using a muffle furnace to fire Al2O3 ceramic shells at 1050°C? Enhance Strength and Purity
- What safety precautions should be taken when using a Muffle furnace? Ensure Lab Safety with Expert Guidelines
- Why is the type of controller important in a muffle furnace? Unlock Precision and Repeatability for Your Lab



















