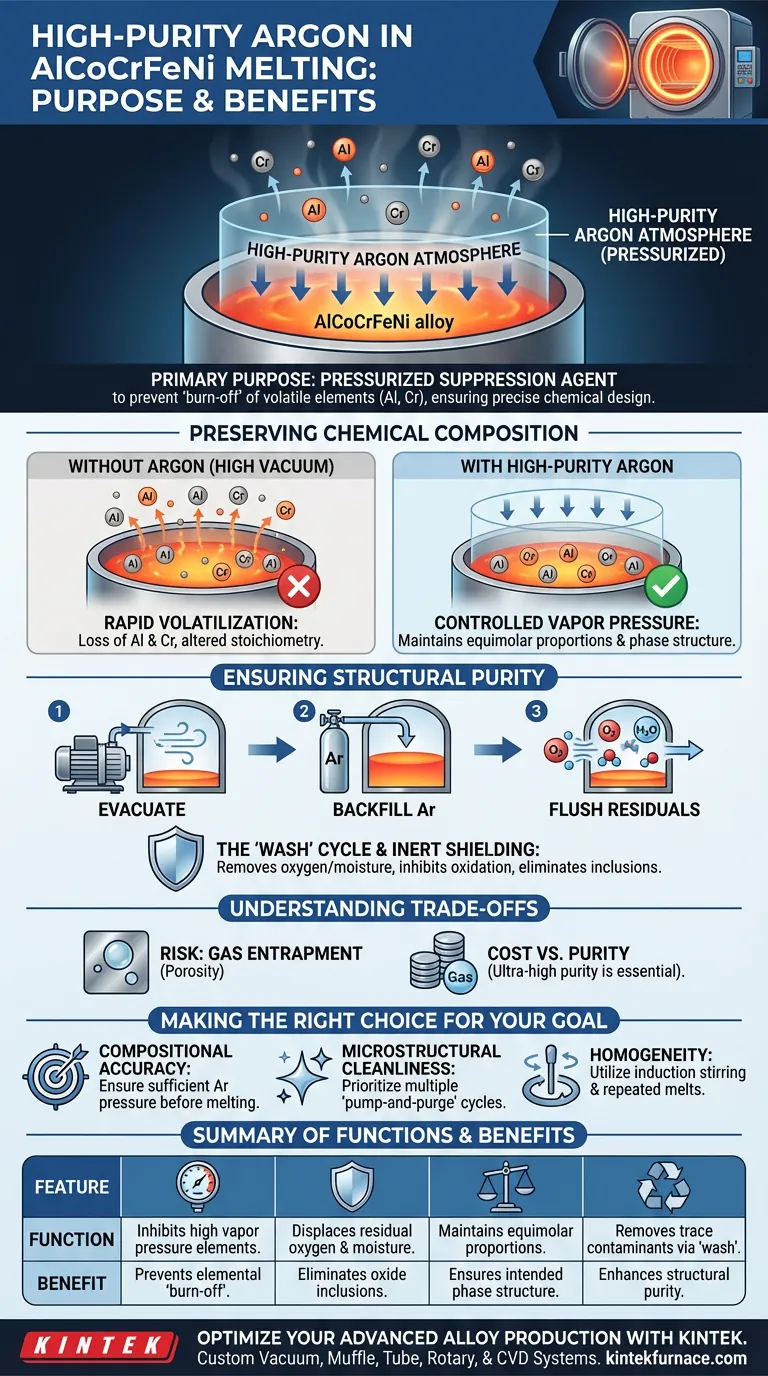
The primary purpose of using a high-purity argon system during the melting of AlCoCrFeNi alloys is to act as a pressurized suppression agent that prevents the loss of volatile elements. While the vacuum furnace removes contaminants, the introduction of argon to reach atmospheric pressure inhibits the "burn-off" of elements with high vapor pressures, ensuring the alloy retains its precise chemical design.
The argon atmosphere is critical for stabilizing the alloy's stoichiometry; without it, reactive components would volatilize in a high vacuum, altering the chemical composition and compromising the intended phase structure of the high-entropy alloy.
Preserving Chemical Composition
Controlling Vapor Pressure
In a high-vacuum environment (such as $10^{-5}$ mbar), the boiling point of certain metals decreases significantly. Elements within the AlCoCrFeNi system, particularly Aluminum (Al) and Chromium (Cr), have relatively high vapor pressures.
Preventing Elemental Burn-off
If the alloy were melted solely under high vacuum, these volatile elements would evaporate or "burn off" rapidly. Introducing high-purity argon creates an atmospheric pressure environment that physically suppresses this evaporation.
Maintaining Equimolar Proportions
High-entropy alloys rely on strict compositional ratios (often equimolar) to achieve their unique properties. By inhibiting volatilization, the argon system ensures the final product matches the designed chemistry, preventing deviations that would alter phase transformation kinetics.
Ensuring Structural Purity
The "Wash" Cycle
Before melting begins, the argon system is often used in a cyclic process: the chamber is evacuated and then backfilled with argon repeatedly. This effectively flushes out residual oxygen and moisture that the vacuum pump alone might not remove from furnace walls.
Inhibiting Oxidation
Aluminum and Chromium are highly reactive and prone to forming oxides instantly upon contact with oxygen. The inert argon atmosphere acts as a protective shield, minimizing the melt's contact with any remaining air.
Eliminating Inclusions
By maintaining extremely low oxygen levels through this inert protection, the process prevents the formation of oxide inclusions. This ensures the structural integrity of the final ingot and prevents defects that could act as failure points.
Understanding the Trade-offs
The Risk of Gas Entrapment
While backfilling with argon preserves composition, it introduces the risk of gas porosity. If the melt acts as a trap for the gas or if solidification occurs too rapidly, argon bubbles can be captured within the metal, creating voids that weaken the material.
Cost vs. Purity
The term "high-purity" is an operational constraint, not just a label. Using standard industrial argon can introduce trace moisture or oxygen, which defeats the purpose of the vacuum system entirely. The cost of ultra-high purity gas is a necessary investment to avoid contaminating the reactive Al and Cr elements.
Making the Right Choice for Your Goal
To maximize the quality of your AlCoCrFeNi alloy, align your process with your specific research or production targets:
- If your primary focus is Compositional Accuracy: Ensure the argon backfill reaches sufficient pressure prior to the melt reaching liquidus temperature to suppress the volatilization of Aluminum.
- If your primary focus is Microstructural Cleanliness: Prioritize multiple "pump-and-purge" cycles with argon before heating to mechanically wash residual oxygen from the chamber walls.
- If your primary focus is Homogeneity: Utilize the induction stirring effect within the argon atmosphere and repeat the melting cycle three times to eliminate chemical segregation.
Control the atmosphere, and you control the alloy's fundamental identity.
Summary Table:
| Feature | Function in AlCoCrFeNi Melting | Benefit |
|---|---|---|
| Pressure Suppression | Inhibits high vapor pressure elements (Al, Cr) | Prevents elemental "burn-off" |
| Inert Shielding | Displaces residual oxygen and moisture | Eliminates oxide inclusions |
| Atmospheric Control | Maintains equimolar proportions | Ensures intended phase structure |
| Cyclic Flushing | Removes trace contaminants via "wash" cycles | Enhances structural purity |
Optimize Your Advanced Alloy Production with KINTEK
Precise control over your thermal environment is the difference between a successful high-entropy alloy and a compromised melt. Backed by expert R&D and manufacturing, KINTEK offers high-performance Vacuum, Muffle, Tube, Rotary, and CVD systems, all fully customizable to meet the rigorous demands of your lab.
Whether you are melting reactive AlCoCrFeNi systems or developing next-generation materials, our high-temp furnaces provide the atmosphere stability and purity you require. Contact us today to discuss your unique needs and see how our tailored solutions can enhance your research and manufacturing efficiency.
Visual Guide
References
- Mudassar Hussain, Tuty Asma Abu Bakar. X-Ray Diffraction Analysis of Sigma-Phase Evolution in Equimolar AlCoCrFeNi High Entropy Alloy. DOI: 10.15282/ijame.21.4.2024.14.0917
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- Why is vacuum sealing in quartz tubes essential for Cr0.82Mn0.18Ge? Ensure Stoichiometry & Purity
- Why are quartz boat properties and cleanliness critical for Si:B nanowires? Ensure High-Purity Synthesis Success
- What are the power specifications for a typical circulating water vacuum pump? Key Specs for Lab Efficiency
- What is the significance of quartz vacuum sealing technology in Dy4T1-xGa12 production? Ensure High-Purity Synthesis
- What is the water-saving benefit of using a water circulating vacuum pump? Save Over 10 Tons of Water Daily
- Why is a laboratory vacuum drying oven utilized for recovered carbon black? Preserve rCB Integrity and Pore Structure
- What is the purpose of an alumina powder bed? Optimize Thermal Debinding for 3D-Printed Ceramic Parts
- What is the importance of using a Mass Flow Controller (MFC)? Enhance Molybdenum Phosphide (MoP) Synthesis Precision
















