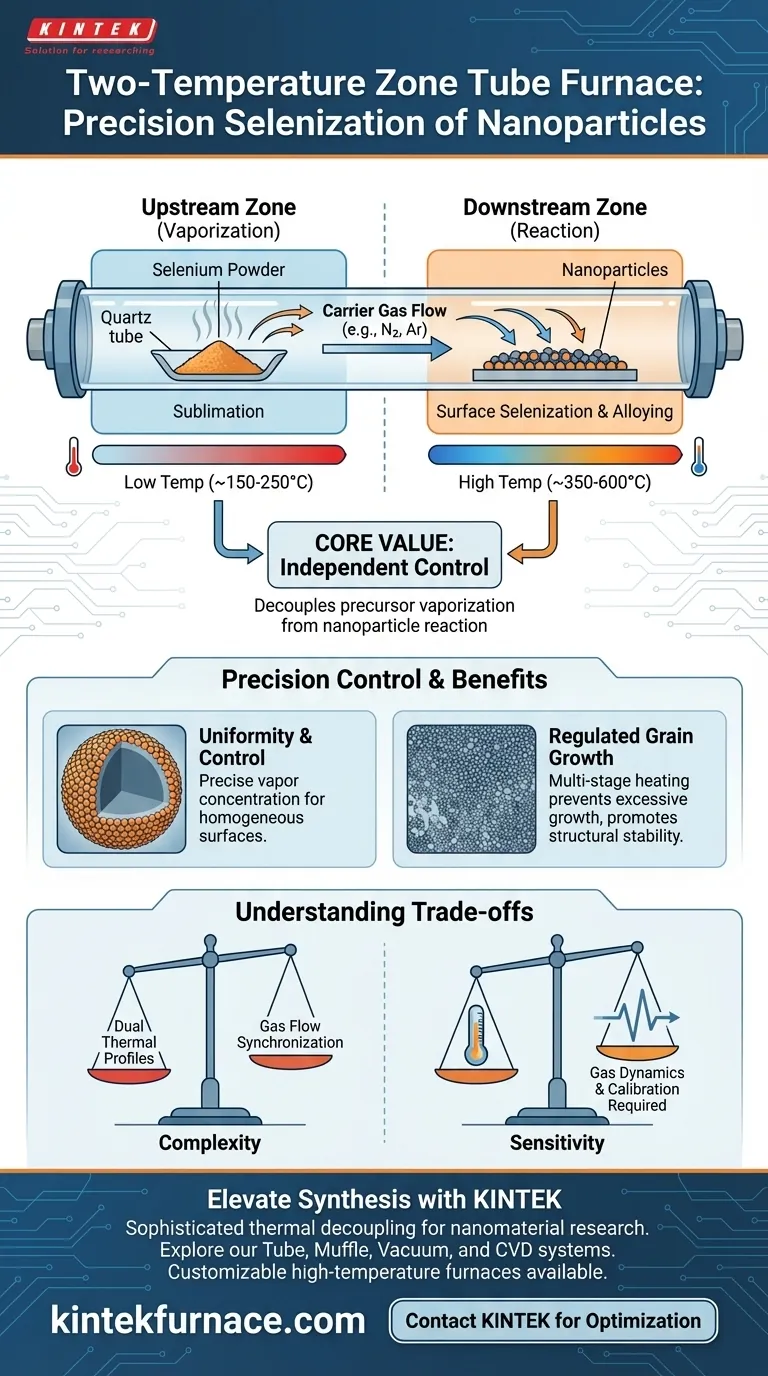
The primary purpose of a two-temperature zone configuration is to physically decouple the vaporization of the selenium precursor from the chemical reaction occurring on the nanoparticle surface. By placing selenium powder in the upstream zone and the sample in the downstream zone, you utilize a precise temperature gradient to sublimate the selenium into vapor before a carrier gas transports it to the high-temperature reaction site.
The core value of this configuration is independent control. It allows you to generate the specific vapor concentration required for uniformity without subjecting the sample to those conditions until the exact moment of reaction, thereby optimizing surface selenization while inhibiting excessive grain growth.

The Mechanics of the Two-Zone System
Spatial Arrangement for Vapor Transport
The fundamental advantage of this setup lies in its physical layout. The selenium powder is positioned upstream, while the target nanoparticles are placed downstream.
This separation is critical because selenium and the target sample often have different thermal requirements. The upstream zone heats the selenium just enough to sublime it, creating a steady stream of vapor.
The Role of the Carrier Gas
Once the selenium sublimates, it does not passively drift to the sample. It is actively carried by a controlled gas flow.
This flow transports the selenium vapor from the first zone to the second, ensuring that the reactant arrives at the sample zone with the correct concentration and velocity.
Precision Control over Material Properties
Ensuring Reaction Uniformity
In a single-zone setup, controlling the exact concentration of vapor at the reaction site is difficult. The two-zone configuration solves this by allowing for precise temperature control in the downstream zone.
This ensures that the selenium vapor reaches the required uniformity at the exact site of the reaction. Consequently, you gain exact control over the degree of selenization on the nanoparticle surfaces.
Regulating Grain Growth and Alloying
High-precision furnaces allow for multi-stage temperature programs (e.g., ramping to 155 °C and then 350 °C). This facilitates the progressive melting and penetration of reactants like sulfur and selenium.
By controlling the heat profile in stages, you promote necessary alloying reactions (such as Ni-S-Se) while simultaneously inhibiting excessive grain growth. This results in ultra-fine, uniformly distributed nanocrystals that are structurally robust.
Understanding the Trade-offs
Process Complexity
While a two-zone furnace offers superior control, it introduces significant complexity to the experimental design. You must manage two distinct thermal profiles and synchronize them with the gas flow rate.
If the upstream temperature is too high relative to the flow rate, you may waste precursor material. If the downstream temperature is misaligned, the vapor may not react efficiently with the sample surface.
Sensitivity to Gas Dynamics
The success of this method relies heavily on the carrier gas. Fluctuations in flow can alter the concentration of selenium vapor reaching the downstream zone.
This sensitivity requires rigorous calibration. You are not just managing heat; you are managing the fluid dynamics of how the vapor travels between the two distinct temperature zones.
Making the Right Choice for Your Goal
To maximize the effectiveness of a two-zone selenization process, consider your specific material objectives:
- If your primary focus is surface uniformity: Prioritize the precise separation of zones to ensure the selenium vapor concentration is homogenous before it contacts the sample.
- If your primary focus is structural stability: Utilize multi-stage temperature programming to facilitate alloying while preventing the formation of overly large grains.
By isolating the vapor generation from the reaction kinetics, you transform selenization from a chaotic thermal event into a tunable, precision-engineered process.
Summary Table:
| Feature | Upstream Zone (Source) | Downstream Zone (Reaction) |
|---|---|---|
| Primary Function | Sublimation of Selenium powder | Chemical reaction on nanoparticles |
| Temperature Role | Controls vapor concentration | Controls reaction kinetics & grain growth |
| Material State | Solid to Vapor transition | Surface alloying & crystallization |
| Key Benefit | Stable precursor delivery | Uniformity & inhibited grain growth |
Elevate Your Nanomaterial Synthesis with KINTEK
Precision in selenization requires more than just heat; it demands the sophisticated thermal decoupling that only a high-performance two-zone system can provide. KINTEK empowers your research with industry-leading Tube, Muffle, Vacuum, and CVD systems, all backed by expert R&D and precision manufacturing.
Whether you need standard configurations or a customizable high-temperature furnace tailored to your unique nanoparticle research, our technical team is ready to assist.
Contact KINTEK today to optimize your laboratory thermal processes.
Visual Guide
References
- Shasha Song, Xingqun Zhu. Synthesis and Lithium Storage Performance of CoO/CoSe Composite Nanoparticles Supported on Carbon Paper. DOI: 10.54691/k2djhp47
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Magnesium Extraction and Purification Condensing Tube Furnace
People Also Ask
- What function does a tube furnace serve in the PVT growth of J-aggregate molecular crystals? Mastery of Thermal Control
- How should crucibles be selected for tube furnaces? Ensure Chemical Purity and Thermal Uniformity
- How is a laboratory tube furnace utilized to convert metal-organic precursors? Master Thin Film Pyrolysis Today
- What is the role of a three-zone vertical furnace in the growth of alpha-Mg3Bi2 single crystals? | KINTEK Solution
- What role do multi-component mass flow controllers play in tube furnace nitrogen studies? Precise Gas Control for NOx.
- What makes fluidized bed vertical tube furnaces environmentally friendly? Discover Efficient Green Tech Solutions
- How does a tubular furnace contribute to the conversion of Co-Fe-ZIF precursors into Co-Fe-NC catalysts?
- What are the different types of tubular furnaces? Choose the Right One for Your Lab



















