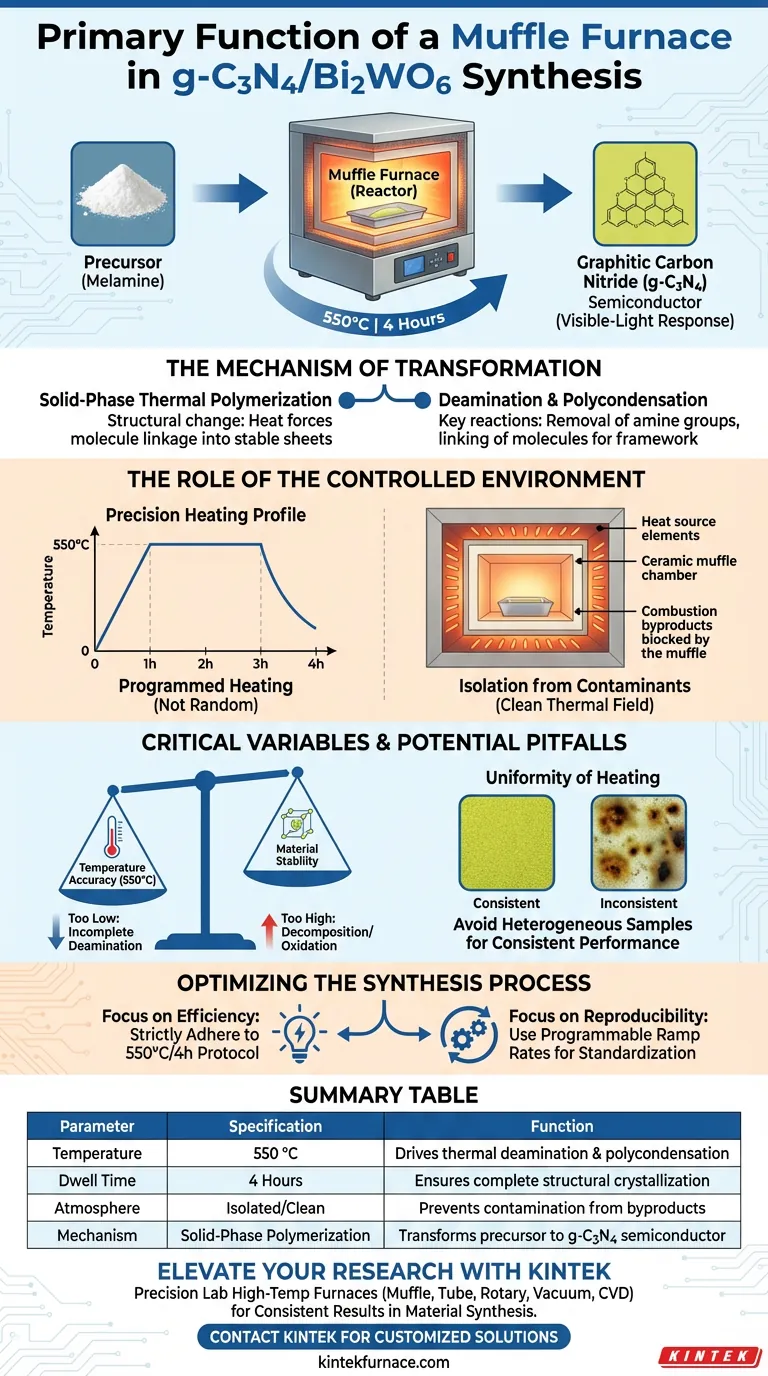
The primary function of a muffle furnace in the preparation of g-C3N4/Bi2WO6 composites is to facilitate the solid-phase thermal polymerization of the precursor material, typically melamine. Through a precisely controlled heating program—specifically maintaining 550 °C for 4 hours—the furnace drives the thermal deamination and polycondensation reactions necessary to transform raw precursors into graphitic carbon nitride (g-C3N4), a semiconductor with essential visible-light response properties.
The muffle furnace acts not merely as a heat source, but as a critical reactor that enables the chemical restructuring of precursors. By isolating the material in a stable, high-temperature environment, it ensures the successful synthesis of the photoactive g-C3N4 component within the composite.

The Mechanism of Transformation
Solid-Phase Thermal Polymerization
The creation of g-C3N4 is a chemical process, not just a physical phase change. The muffle furnace provides the energy required to initiate solid-phase thermal polymerization.
During this phase, the precursor (melamine) undergoes significant structural changes. The heat forces the molecules to link together, forming the stable graphitic sheets that define the material.
Deamination and Polycondensation
Two specific chemical reactions occur within the furnace: thermal deamination (removal of amine groups) and polycondensation (linking of molecules releasing small byproducts).
These reactions are responsible for building the specific atomic framework of the g-C3N4 semiconductor. Without the sustained high energy provided by the furnace, these reactions would not reach completion, resulting in a material with poor electronic properties.
The Role of the Controlled Environment
Precision Heating Profile
The synthesis relies on programmed heating, not random thermal exposure. The standard protocol requires maintaining the environment at 550 °C for 4 hours.
This specific duration and temperature window are critical. They allow enough time for the polymeric structure to form fully without degrading the material.
Isolation from Contaminants
A defining feature of a muffle furnace is its ability to separate the workload from the combustion byproducts of the heat source.
This isolation creates a "clean" thermal field. It ensures that the g-C3N4/Bi2WO6 composite is not contaminated by external gases or particulate matter during the sensitive crystallization and polymerization stages.
Critical Variables and Potential Pitfalls
While the muffle furnace is a robust tool, its effectiveness depends on the management of key variables.
Temperature Accuracy vs. Material Stability
The relationship between temperature and material integrity is delicate.
- Too Low: If the temperature fails to reach or maintain 550 °C, the deamination process will be incomplete, leading to a defective crystal structure.
- Too High: Excessive heat can cause the decomposition of the g-C3N4 structure or unwanted oxidation, destroying the semiconductor properties you are trying to create.
Uniformity of Heating
Inconsistent heating within the furnace chamber can lead to heterogeneous samples. If one part of the composite is sintered while another is under-reacted, the final material will exhibit inconsistent photocatalytic performance.
Optimizing the Synthesis Process
To ensure high-quality g-C3N4/Bi2WO6 composites, you must tailor your furnace usage to your specific goals.
- If your primary focus is Photocatalytic Efficiency: Strictly adhere to the 550 °C/4-hour protocol to ensure complete polymerization and maximum visible-light response.
- If your primary focus is Reproducibility: Use a furnace with programmable ramp rates to standardize the heating and cooling cycles across every batch.
The muffle furnace is the foundational tool that dictates whether your raw chemicals become a high-performance semiconductor or merely burnt powder.
Summary Table:
| Parameter | Specification | Function in Synthesis |
|---|---|---|
| Temperature | 550 °C | Drives thermal deamination and polycondensation |
| Dwell Time | 4 Hours | Ensures complete structural crystallization |
| Atmosphere | Isolated/Clean | Prevents contamination from combustion byproducts |
| Mechanism | Solid-Phase Polymerization | Transforms melamine precursor into g-C3N4 semiconductor |
Elevate Your Photocatalytic Research with KINTEK
Precision is the difference between a high-performance semiconductor and a failed experiment. Backed by expert R&D and world-class manufacturing, KINTEK offers specialized Muffle, Tube, Rotary, Vacuum, and CVD systems designed for sensitive material synthesis.
Whether you are preparing g-C3N4/Bi2WO6 composites or developing next-generation catalysts, our lab high-temp furnaces provide the uniform heating and programmable control required for consistent results. Our systems are fully customizable to meet your unique laboratory needs.
Ready to optimize your synthesis process? Contact KINTEK today for a customized solution!
Visual Guide
References
- Wenxing Chen, Huilin Hou. Engineering g-C3N4/Bi2WO6 Composite Photocatalyst for Enhanced Photocatalytic CO2 Reduction. DOI: 10.3390/coatings15010032
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How does the muffle in a muffle furnace protect samples? Ensure Purity and Uniformity in Thermal Processing
- What are the common applications of box furnaces? Versatile Heat Treatment for Metals, Ceramics, and Research
- What are the technical advantages of using an infrared heating furnace for the fast pyrolysis of cellulose? Higher Yields
- How does a muffle furnace compare to other types of laboratory melting furnaces? Discover the Best Fit for Your Lab
- What temperature precautions should be observed when using a muffle furnace? Ensure Safety and Longevity in Your Lab
- How does a programmable muffle furnace facilitate lithium disilicate crystallization? Master Ceramic Heat Treatments
- How did electric heating elements change Muffle Furnace design? Revolutionizing Precision and Clean Heating
- What material properties can be achieved using a box furnace? Unlock Enhanced Hardness, Strength, and More



















