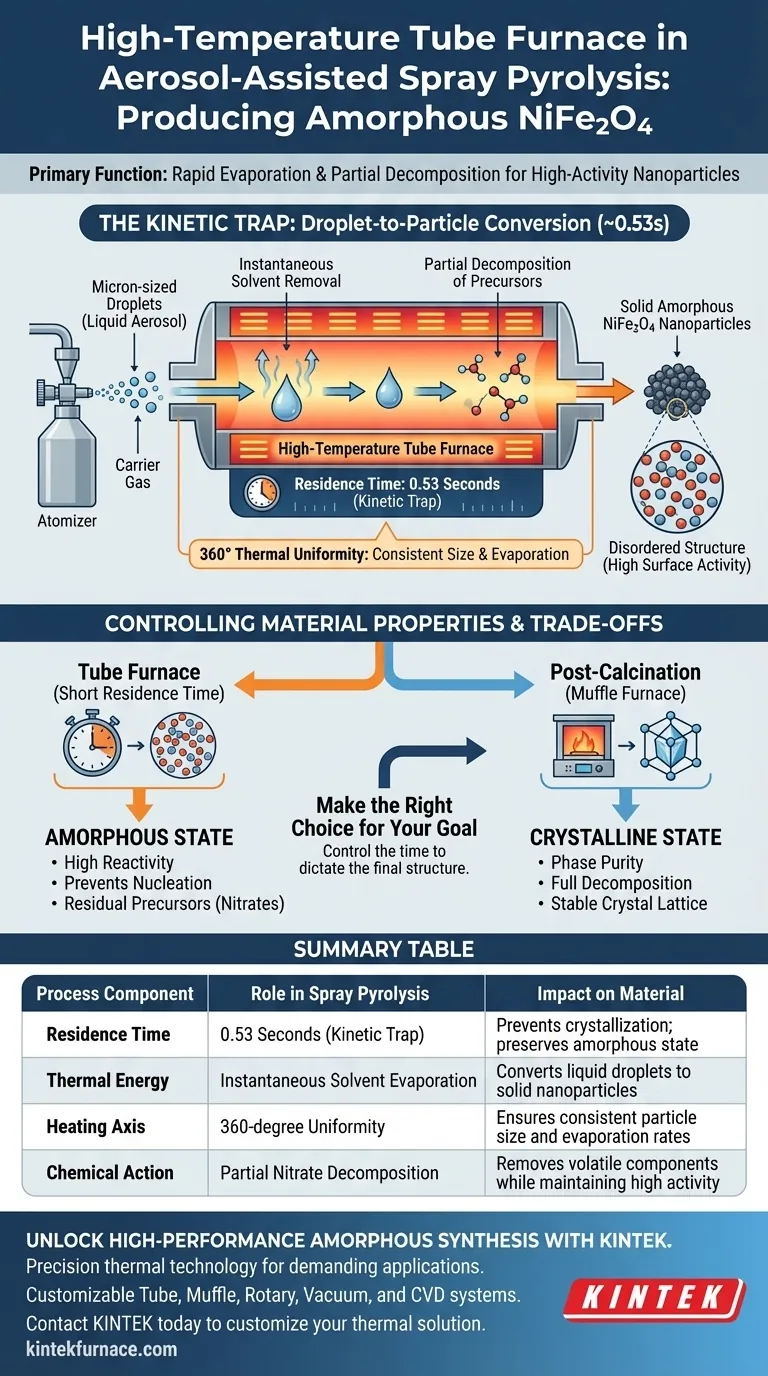
The primary function of the high-temperature tube furnace in aerosol-assisted spray pyrolysis is to facilitate the rapid evaporation of water and the partial decomposition of metal nitrates. By maintaining a specific temperature and a very short residence time, the furnace drives a "droplet-to-particle" conversion that creates solid nanoparticles instantly. This process is engineered to produce highly active amorphous materials rather than crystalline structures.
The tube furnace operates as a kinetic trap, utilizing a residence time of approximately 0.53 seconds to dry and decompose droplets before atoms can organize into a crystal lattice. This rapid thermal shock is the defining mechanism for generating amorphous NiFe2O4.

The Mechanism of Rapid Conversion
Instantaneous Solvent Removal
The furnace receives micron-sized droplets generated by an atomizer and transported by a carrier gas. Upon entering the heated zone, the thermal energy causes the immediate evaporation of the water solvent within the droplets. This transforms the liquid aerosol into solid precursors in a fraction of a second.
Partial Decomposition of Precursors
Simultaneously, the furnace heat triggers the partial decomposition of the metal nitrates contained within the droplets. This chemical breakdown is essential for removing volatile components. However, because the exposure to heat is brief, the decomposition is not intended to be chemically exhaustive at this stage, but rather sufficient to form the solid particle structure.
Thermal Uniformity
While the reaction is fast, the quality of the product depends on the tube furnace's ability to provide consistent heating. The cylindrical heating elements ensure that heat is distributed evenly across a 360-degree axis. This prevents temperature gradients that could lead to uneven evaporation rates or inconsistent particle sizes.
Controlling Material Properties
The Critical Role of Residence Time
The defining characteristic of this process is the residence time, specifically cited as 0.53 seconds. This duration is strictly controlled by the gas flow rate and the length of the heated zone. It provides just enough energy to form the particle but not enough time for the material to reach thermodynamic equilibrium.
Preserving the Amorphous State
In standard solid-state synthesis, heat is used to provide activation energy for crystal growth. In this specific application, however, the goal is the opposite. The rapid heating and short duration prevent the nucleation and growth of crystals, locking the NiFe2O4 into an amorphous (non-crystalline) state which often exhibits higher surface activity.
Understanding the Trade-offs
Amorphous Activity vs. Crystalline Stability
The tube furnace, when used in this specific manner, optimizes for high reactivity (amorphous structure) at the expense of structural order. If your application requires a stable, fully crystalline spinel structure, this step alone is insufficient.
Residual Precursors
Because the residence time is so short, the decomposition of nitrates is only partial. The resulting nanoparticles may contain residual nitrate groups. To achieve a pure, highly crystalline phase, a secondary step involving a muffle furnace (post-calcination) would be required to fully decompose these residues and promote crystal growth.
Making the Right Choice for Your Goal
To optimize your production of NiFe2O4, align your thermal treatment with your desired material properties:
- If your primary focus is high catalytic activity: Prioritize the short residence time (~0.53s) in the tube furnace to maintain the amorphous structure and prevent crystal lattice formation.
- If your primary focus is phase purity and crystallinity: View the tube furnace product as an intermediate precursor that requires subsequent post-calcination in a muffle furnace to fully remove nitrates and grow crystals.
Control the time, not just the temperature, to dictate the final structure of your material.
Summary Table:
| Process Component | Role in Spray Pyrolysis | Impact on Material |
|---|---|---|
| Residence Time | 0.53 Seconds (Kinetic Trap) | Prevents crystallization; preserves amorphous state |
| Thermal Energy | Instantaneous Solvent Evaporation | Converts liquid droplets to solid nanoparticles |
| Heating Axis | 360-degree Uniformity | Ensures consistent particle size and evaporation rates |
| Chemical Action | Partial Nitrate Decomposition | Removes volatile components while maintaining high activity |
Unlock High-Performance Amorphous Synthesis with KINTEK
Precision is the difference between a crystalline structure and a high-activity amorphous material. KINTEK provides the advanced thermal technology required for demanding applications like aerosol-assisted spray pyrolysis. Backed by expert R&D and manufacturing, we offer high-precision Tube, Muffle, Rotary, Vacuum, and CVD systems—all fully customizable to meet your specific residence time and temperature uniformity needs.
Whether you are scaling NiFe2O4 production or researching novel catalysts, our lab high-temp furnaces ensure the control you need to dictate material properties.
Contact KINTEK today to customize your thermal solution
Visual Guide
References
- Jan Witte, Thomas Turek. Efficient Anion Exchange Membrane Water Electrolysis on Amorphous Spray‐Pyrolyzed NiFe<sub>2</sub>O<sub>4</sub>. DOI: 10.1002/celc.202500226
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is an atmosphere tube furnace? Unlock Precise High-Temperature Processing
- What are the specifications for large volume single zone tube furnaces? Find Your Ideal High-Temp Solution
- What critical process conditions does a horizontal diffusion furnace provide? Master Silicide Formation Today
- What is the role of a Tube Furnace or Rotary Furnace in hydrogen reduction roasting? Optimize Lithium Recovery Efficiency.
- What is a vertical tube furnace and how does it function? Optimize Material Processing with Precision
- What is sintering, and how is it performed in horizontal furnaces? Unlock Precision in Powder Processing
- How is heat transferred to the material inside a tube furnace? Master the 3-Stage Process for Precise Thermal Control
- How does a tube atmosphere furnace facilitate local CVD during PAN fiber carbonization? Master In-Situ CNT Growth



















