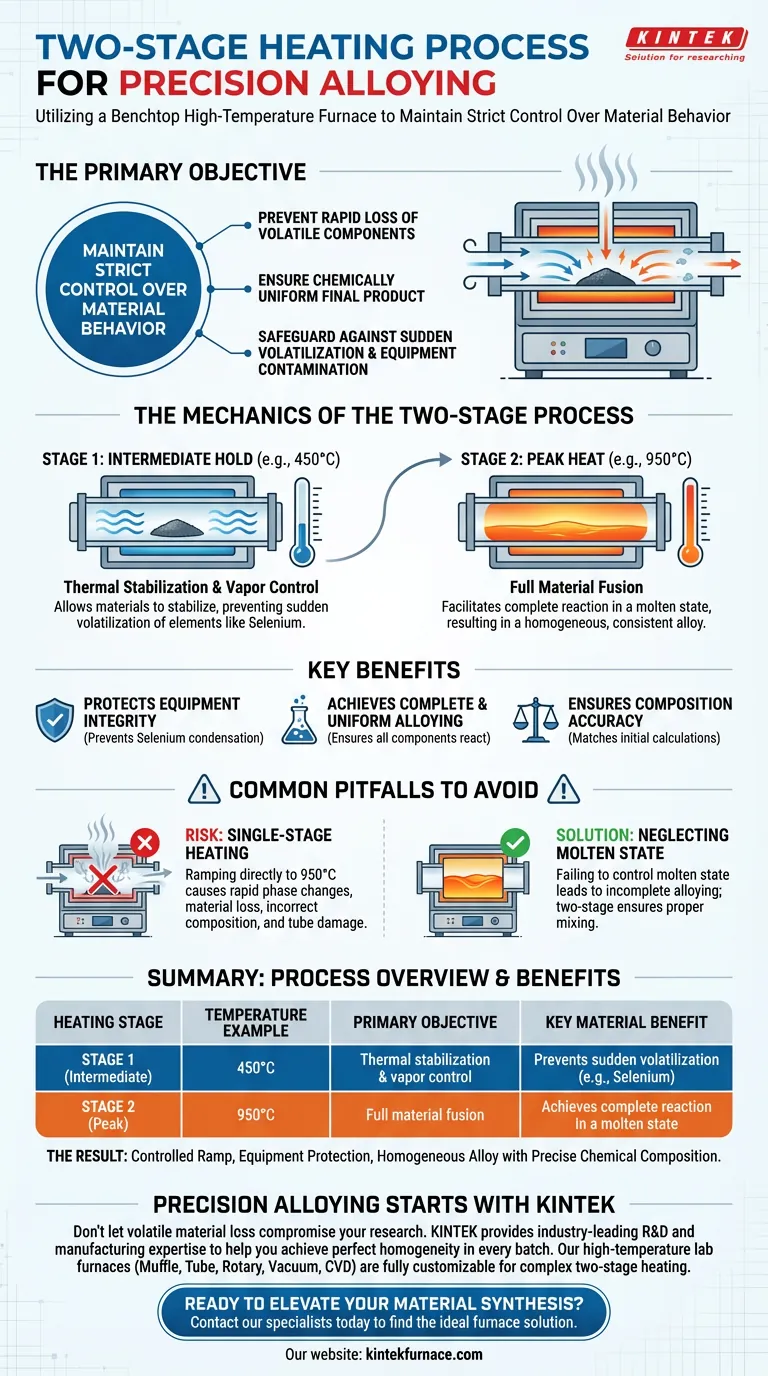
The primary objective of utilizing a two-stage heating process in a benchtop high-temperature furnace is to maintain strict control over material behavior during the alloying phase. By implementing a thermal hold at an intermediate temperature (such as 450°C) before ascending to the final target (950°C), the system prevents the rapid loss of volatile components and ensures a chemically uniform final product.
A two-stage heating profile acts as a critical safeguard against sudden volatilization and equipment contamination. By stabilizing the reaction environment, it forces all elements to react fully in a molten state, ensuring the integrity of the alloy.

The Mechanics of the Two-Stage Process
Preventing Sudden Volatilization
In high-temperature alloying, rapid heating can cause unstable components to evaporate immediately rather than mix.
A two-stage process mitigates this by holding the material at a lower, intermediate temperature (e.g., 450°C). This allows the materials to stabilize thermally before they are subjected to the peak heat (e.g., 950°C), effectively preventing sudden volatilization.
Protecting Equipment Integrity
One of the specific risks in this process is the behavior of volatile elements like selenium.
Without a controlled heating path, selenium vapor can condense on the cooler parts of the tube walls. This not only results in the loss of material from the alloy but also contaminates the furnace setup. The two-stage approach keeps these elements within the reaction zone.
Achieving Complete and Uniform Alloying
The ultimate goal of the process is reaction efficiency.
By preventing the escape of volatile elements, the furnace ensures that all components remain available to react. This facilitates a complete reaction within the molten state, resulting in a final alloy that is homogeneous and consistent.
Common Pitfalls to Avoid
The Risk of Single-Stage Heating
Attempting to ramp directly to the final temperature (950°C) is a common error that compromises the material.
Skipping the intermediate hold triggers rapid phase changes that lead to material loss through evaporation. This results in an alloy with an incorrect chemical composition and potentially damages the furnace tube via condensation.
Neglecting the Molten State Reaction
If the heating profile is too aggressive, components may separate before they can mix.
The two-stage process ensures that the reaction occurs while the materials are properly molten. Failing to control this state leads to incomplete alloying, where the final product lacks the intended physical and chemical properties.
Making the Right Choice for Your Goal
To ensure your alloying process yields the highest quality results, consider the following specific applications of this heating strategy:
- If your primary focus is Composition Accuracy: Prioritize the intermediate hold at 450°C to prevent the loss of volatile components like selenium, ensuring your final ratio matches your initial calculation.
- If your primary focus is Process Consistency: Utilize the two-stage profile to enforce a complete reaction in the molten state, guaranteeing that every batch achieves the same level of uniformity.
Control the temperature path precisely, and you secure both the purity of your alloy and the longevity of your equipment.
Summary Table:
| Heating Stage | Temperature Example | Primary Objective | Key Material Benefit |
|---|---|---|---|
| Stage 1 (Intermediate) | 450°C | Thermal stabilization & vapor control | Prevents sudden volatilization of elements like Selenium |
| Stage 2 (Peak) | 950°C | Full material fusion | Achieves complete reaction in a molten state |
| The Result | Controlled Ramp | Equipment protection | Homogeneous alloy with precise chemical composition |
Precision Alloying Starts with KINTEK
Don't let volatile material loss compromise your research. KINTEK provides industry-leading R&D and manufacturing expertise to help you achieve perfect homogeneity in every batch. Whether you require Muffle, Tube, Rotary, Vacuum, or CVD systems, our high-temperature lab furnaces are fully customizable to support complex two-stage heating profiles and protect your equipment from contamination.
Ready to elevate your material synthesis? Contact our specialists today to find the ideal furnace solution for your unique alloying needs.
Visual Guide
References
- Mohamed Muthana Ghazi, Kareem A. Jasim. An investigation into the implications of partial substitution of selenium with lead on the thermal properties for S<sub>60</sub>Se<sub>40-X</sub>Pb<sub>X</sub> Chalcogenide Compound. DOI: 10.1088/1742-6596/2857/1/012007
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What design features enhance the versatility of a box furnace? Boost Your Lab's Thermal Processing Capabilities
- What is the main purpose of a muffle furnace? Achieve Contamination-Free High-Temperature Heating
- How does a high-temperature laboratory box furnace facilitate the synthesis of Ba7Nb4MoO20? Achieve Phase Purity
- What role does a high-temperature experimental furnace play in sintering Li2Mg3Ti(1-x)ZrxO6 ceramics?
- What is the role of a laboratory box muffle furnace in the compositional analysis of finger millet popcorn?
- What principles do muffle furnaces operate on? Master Heat, Isolation, and Uniform Transfer
- What are the different types of muffle furnaces based on appearance and shape? Choose the Right Furnace for Your Lab
- What materials are used in a box type resistance furnace's structure? Discover the Key Materials for Durability and Efficiency



















