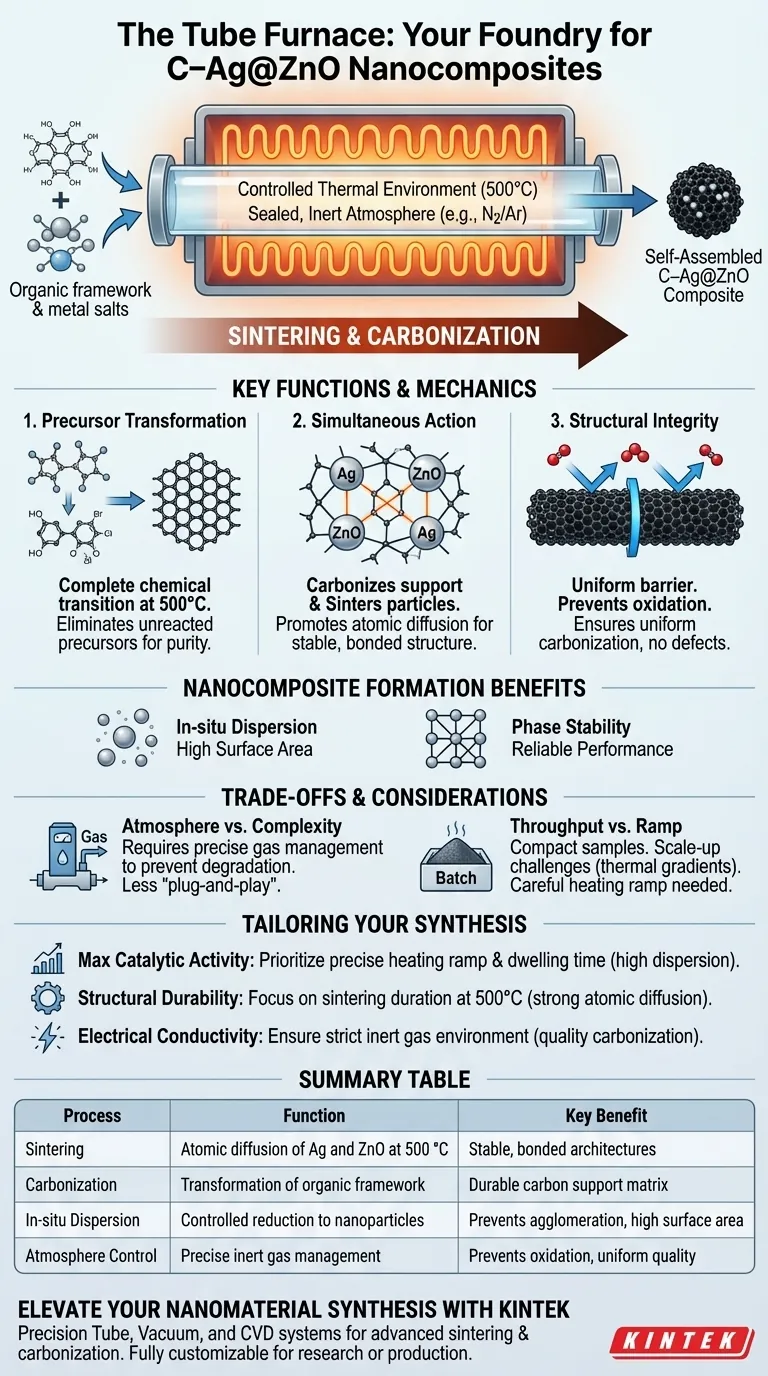
The tube furnace serves as the primary reactor for sintering and carbonization during the final synthesis stage. By providing a strictly controlled thermal environment at 500 °C, the furnace facilitates the complete transformation of precursors into a self-assembled carbon-supported silver-zinc oxide (C–Ag@ZnO) composite. Its sealed design is crucial for ensuring uniform carbonization and maintaining the structural integrity of the resulting nanomaterial.
The tube furnace is the "foundry" for the nanocomposite, enabling the simultaneous carbonization of the organic framework and the sintering of the silver-zinc oxide components into a stable, functional structure.

The Role of Controlled Thermal Processing
Achieving Complete Precursor Transformation
The primary function of the furnace is to drive the chemical transition from raw precursors to the final C–Ag@ZnO state. At the specific threshold of 500 °C, the organic components begin to decompose and rearrange into a carbon matrix. This high-temperature environment ensures that no unreacted precursors remain, which would otherwise compromise the purity of the composite.
Facilitating Carbonization and Sintering
The furnace performs two tasks simultaneously: it carbonizes the support structure and sinters the metal/oxide particles. Sintering promotes atomic diffusion, which helps in bonding the silver (Ag) and zinc oxide (ZnO) particles together. This process creates a "self-assembled" architecture where the active materials are securely anchored within the carbon support.
Maintaining Structural Integrity
The sealed structure of a tube furnace is a technical necessity rather than a convenience. It prevents the influx of oxygen, which would cause the carbon support to burn away rather than carbonize. This containment ensures that the carbonization is uniform across the entire sample, preventing structural defects or weak points in the final composite.
Mechanics of Nanocomposite Formation
In-situ Nanoparticle Dispersion
During the heating phase, the furnace enables in-situ formation, where metal salts are reduced into highly dispersed nanoparticles. Because the temperature is controlled precisely, the silver nanoparticles do not clump together (agglomerate). This results in a high surface area, which is vital for the material’s eventual performance in catalytic or electronic applications.
Promoting Atomic Diffusion and Phase Stability
The thermal energy provided by the furnace facilitates atomic diffusion between the powder particles. This eliminates residual stresses that may have been introduced during the initial mixing or pressing stages of synthesis. By maintaining a steady temperature, the furnace allows the zinc oxide and silver to reach a stable phase, ensuring the material does not degrade during use.
Understanding the Trade-offs
Atmosphere Control vs. Process Complexity
While the tube furnace offers a precise inert or controlled atmosphere, it requires careful management of gas flow (such as nitrogen or argon). If the seal is compromised or the gas purity is low, the silver may oxidize, or the carbon matrix may undergo thermo-oxidative degradation. This makes the setup more complex and less "plug-and-play" than standard muffle furnaces.
Throughput and Heating Ramp Limitations
Tube furnaces are generally designed for compact samples and research-scale batches. Achieving a uniform heating ramp (e.g., 1 °C per minute) is easier in a small tube, but scaling this process for mass production introduces challenges in maintaining thermal gradients. Rapid heating can lead to "low shrinkage" issues or internal stresses, while too slow a ramp may lead to unwanted grain growth.
Applying This to Your Synthesis Goals
The use of a tube furnace must be tailored to the specific functional requirements of your C–Ag@ZnO composite.
- If your primary focus is maximum catalytic activity: Prioritize a precise heating ramp and dwelling time to ensure silver nanoparticles remain highly dispersed and do not undergo excessive grain growth.
- If your primary focus is structural durability: Focus on the sintering duration at 500 °C to promote stronger atomic diffusion and grain boundary bonding between the ZnO and the carbon framework.
- If your primary focus is electrical conductivity: Ensure the sealed environment is strictly maintained with an inert gas like nitrogen to maximize the quality of the carbonization process.
The tube furnace is the defining tool that transforms a mixture of chemicals into a sophisticated, high-performance nanocomposite through the synergy of heat and atmosphere control.
Summary Table:
| Process Function | Description | Key Benefit |
|---|---|---|
| Sintering | Atomic diffusion of Ag and ZnO at 500 °C | Creates stable, bonded architectures |
| Carbonization | Transformation of organic framework in sealed environment | Forms a durable carbon support matrix |
| In-situ Dispersion | Controlled reduction of metal salts into nanoparticles | Prevents agglomeration for high surface area |
| Atmosphere Control | Precise management of inert gas flow (N2/Ar) | Prevents oxidation and ensures uniform quality |
Elevate Your Nanomaterial Synthesis with KINTEK
Precision is paramount when synthesizing complex C–Ag@ZnO nanocomposites. KINTEK provides industry-leading Tube, Vacuum, and CVD systems designed to deliver the exact thermal environments and atmosphere control required for advanced carbonization and sintering.
Backed by expert R&D and manufacturing, our laboratory high-temperature furnaces are fully customizable to meet your unique research or production needs. Ensure structural integrity and maximum catalytic performance with equipment you can trust.
Ready to optimize your synthesis process? Contact us today to find the perfect thermal solution!
Visual Guide
References
- Parameswari R. Nithiasri, B. Karthikeyan. Novel self-assembled valine-derived carbon-supported Ag@ZnO optical materials for enhanced photodegradation and anti-bacterial activity. DOI: 10.1039/d5na00427f
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What is the mechanism by which the presulfidation process influences the coking behavior of cracking furnace tubes?
- Why is precise heating rate control in a high-temperature tube furnace critical for HyDR? Master Reduction Kinetics
- Why is an Ultra-High Vacuum (UHV) compatible tube furnace necessary for beta-Ga2O3? Protect Your Semiconductor Integrity
- In what scenarios are laboratory high-temperature tube furnaces or muffle furnaces utilized? Study MgTiO3-CaTiO3 Ceramics
- What are the advantages of using infrared-heated SiC tube furnaces for Zirconia phase transformations? Expert Guide
- What factors should be considered when purchasing a drop tube furnace? Key specs for precision and efficiency
- Why is a tube furnace with precise temperature control required for Pt@A&R-TiO2 calcination? Optimize Catalyst Phase
- How does a high-temperature tube furnace form Nitrogen-doped Porous Carbon (RMF)? Precision Thermal Synthesis Guide



















