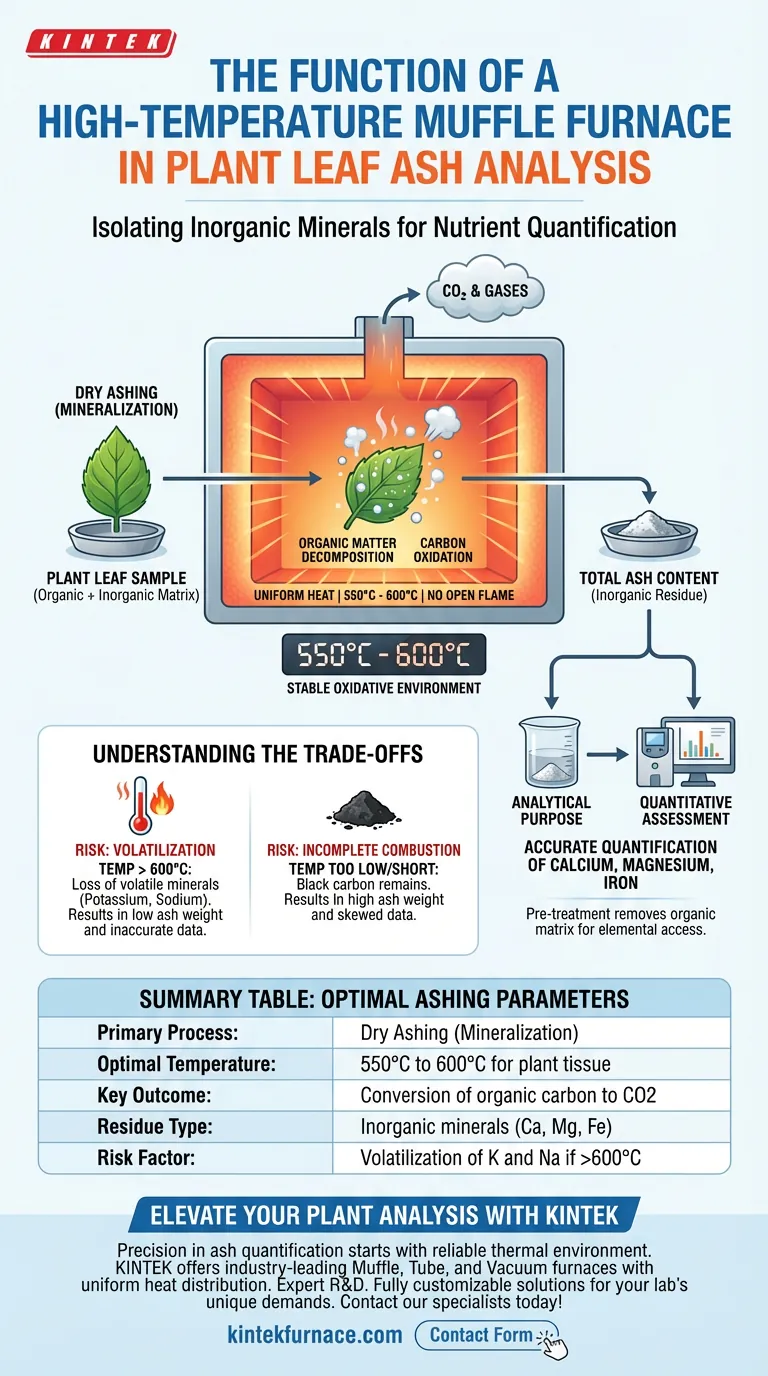
The primary function of a high-temperature muffle furnace is to isolate inorganic minerals from plant tissue through controlled incineration. By subjecting plant leaves to a stable, high-heat environment, the furnace completely oxidizes organic matter, converting carbon into carbon dioxide and leaving behind only the mineral residue. This process, often called mineralization or dry ashing, is a fundamental prerequisite for determining total ash content.
The muffle furnace acts as a precision oxidizer, stripping away organic complexity to reveal the elemental baseline. It ensures that only inorganic residues remain, enabling the accurate quantification of nutrients like calcium, magnesium, and iron without interference from carbon compounds.

The Mechanism of Mineralization
Creating a Stable Oxidative Environment
The furnace generates a consistent, high-temperature atmosphere, typically maintained around 550°C to 600°C for plant materials.
Unlike an open flame, the muffle furnace surrounds the sample with radiant heat. This ensures uniform temperatures throughout the chamber, preventing hot spots that could cause uneven burning or sample loss.
Conversion of Carbon to Gas
The core chemical reaction driven by the furnace is oxidation.
The high heat causes the organic components of the leaves (cellulose, lignin, proteins) to decompose. The carbon within these structures reacts with oxygen to form carbon dioxide, which is expelled from the furnace as gas.
Isolation of Inorganic Residue
Once the organic matter is volatilized, the only substance remaining is the total ash.
This residue consists entirely of inorganic minerals that were absorbed by the plant during its life cycle. This separation is the "dry ashing" technique, transforming a complex biological sample into a simple mineral powder.
The Analytical Purpose
Pre-treatment for Nutrient Analysis
Isolating the ash is rarely the final goal; it is a critical preparation step.
Analytical instruments cannot easily measure specific elements while they are bound inside complex organic plant structures. The furnace removes the organic matrix, making the minerals accessible for downstream analysis.
Quantitative Assessment
The resulting ash allows researchers to calculate the total mineral percentage by mass.
By weighing the sample before and after incineration, analysts can determine the precise fraction of the leaf composed of minerals. This ash is then often dissolved in acid to quantify specific nutrients such as calcium, magnesium, and iron.
Understanding the Trade-offs
While the muffle furnace is the standard tool for total ash analysis, the process requires careful management to avoid analytical errors.
The Risk of Volatilization
If the temperature is set too high (exceeding 600°C for certain plant tissues), you risk losing volatile minerals.
Elements such as potassium or sodium can vaporize at extreme temperatures. This results in an artificially low ash weight and inaccurate nutrient data.
Incomplete Combustion
If the temperature is too low or the duration too short, black carbon residues will remain.
This indicates that the organic matter was not fully oxidized. The resulting "ash" will weigh more than it should, skewing the total ash content calculation and interfering with chemical quantification.
Making the Right Choice for Your Goal
To ensure data integrity, you must tailor the furnace parameters to your specific analytical targets.
- If your primary focus is Total Ash Quantification: Ensure the final residue is a clean white or grey powder, indicating that all carbon has been successfully converted to CO2.
- If your primary focus is Volatile Trace Elements: strictly limit the upper temperature (typically to 550°C) to prevent the thermal loss of sensitive inorganic components.
A properly calibrated muffle furnace transforms biological chaos into chemical clarity.
Summary Table:
| Feature | Specification/Role |
|---|---|
| Primary Process | Dry Ashing (Mineralization) |
| Optimal Temperature | 550°C to 600°C for plant tissue |
| Key Outcome | Conversion of organic carbon to CO2 |
| Residue Type | Inorganic minerals (Calcium, Magnesium, Iron) |
| Risk Factor | Volatilization of K and Na if >600°C |
Elevate Your Plant Analysis with KINTEK
Precision in ash quantification starts with a reliable thermal environment. KINTEK provides industry-leading Muffle, Tube, and Vacuum furnaces designed to deliver the uniform heat distribution essential for accurate mineralization.
Backed by expert R&D and manufacturing, our systems are fully customizable to meet the unique demands of your lab. Whether you are analyzing volatile trace elements or determining total mineral mass, KINTEK ensures your data integrity through superior temperature control.
Contact our specialists today to find your custom furnace solution!
Visual Guide
References
- Effects of Drying Temperatures on Nutritional and Phytochemical Properties of Gongronema Latifolium Leaves. DOI: 10.63958/azojete/2025/21/2/001
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How does a laboratory high-temperature box furnace ensure the material performance of NN-10ST based ceramics?
- What is a muffle furnace used for? Achieve Pure, High-Temperature Processing
- What are some important 'Do's' when operating a muffle furnace? Ensure Safety and Efficiency in Your Lab
- How should the temperature controller be set up before using the muffle furnace? Ensure Safe and Accurate Heating
- What are the main benefits of using a muffle furnace? Achieve Precise, Contamination-Free Heating for Your Lab
- How are Box Furnaces typically loaded? Manual Methods for Flexible Batch Processing
- What role does a high-temperature box furnace play in the secondary sintering and structural repair of recycled NCM?
- What are the key safety measures to prioritize when working with benchtop furnaces? Ensure Safe Operation with Expert Guidelines



















