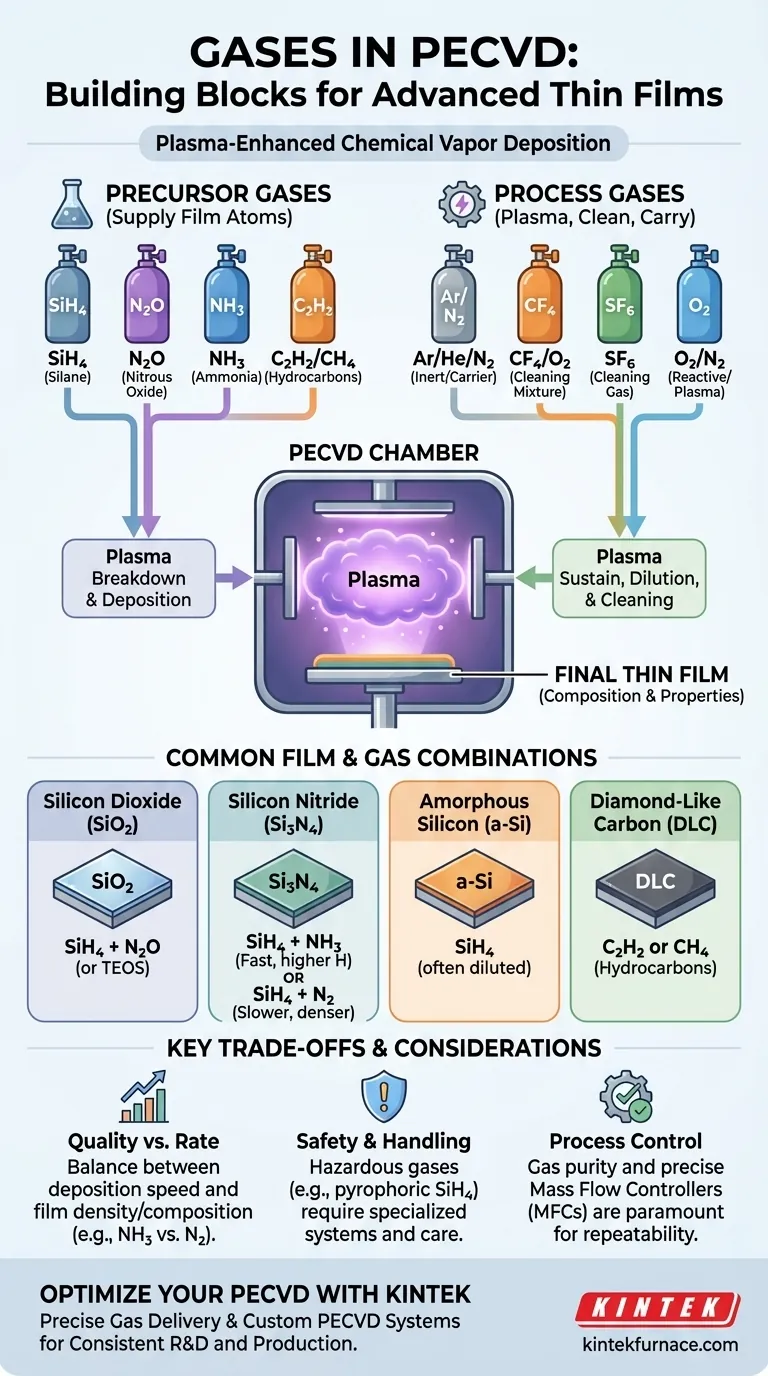
In Plasma-Enhanced Chemical Vapor Deposition (PECVD), the gases used are a combination of precursors, which supply the atoms for the film, and process gases, which help generate the plasma or clean the chamber. Common precursor gases include silane (SiH4) for silicon, ammonia (NH3) or nitrogen (N2) for nitrogen, and nitrous oxide (N2O) for oxygen. Process gases include inert carriers like argon (Ar) and helium (He), and cleaning gases like sulfur hexafluoride (SF6) or a CF4/O2 mixture.
The specific gases chosen for a PECVD process are not arbitrary; they are the fundamental ingredients that directly determine the chemical composition, structure, and properties of the final thin film being deposited on the substrate.

The Role of Precursor Gases
The core of PECVD is using plasma to break down source gases, known as precursors, into reactive species that then deposit onto a substrate. The precursor gas must contain the element you intend to deposit.
Silicon-Based Films (SiO₂, Si₃N₄, a-Si)
This is the most common application of PECVD, especially in microelectronics for creating insulating and semiconductor layers.
- For Silicon Dioxide (SiO₂): The process typically combines a silicon source like silane (SiH₄) with an oxygen source, most commonly nitrous oxide (N₂O). Tetraethyl orthosilicate (TEOS) can also be used as a liquid silicon source.
- For Silicon Nitride (Si₃N₄): A silicon source like SiH₄ is combined with a nitrogen source. Ammonia (NH₃) is frequently used, though pure nitrogen (N₂) can also be employed for films with lower hydrogen content.
- For Amorphous Silicon (a-Si): This requires only a silicon source gas, which is almost always silane (SiH₄). It may be diluted with hydrogen or argon.
- For Silicon Oxynitride (SiOxNy): The properties of this film can be tuned by flowing a mixture of all three precursors: SiH₄, N₂O, and NH₃. The gas flow ratios determine the final refractive index and stoichiometry.
Carbon-Based and Polymer Films
PECVD is also highly effective for creating hard, protective coatings and specialized polymers.
- For Diamond-Like Carbon (DLC): These super-hard, low-friction coatings are deposited using hydrocarbon gases like acetylene (C₂H₂) or methane (CH₄).
- For Polymer Films: A wide range of organic and inorganic polymers can be deposited. This includes fluorocarbons for creating hydrophobic surfaces and silicones for biocompatible coatings.
Understanding Process and Carrier Gases
Not every gas that enters the chamber becomes part of the final film. Many gases serve critical process functions.
Carrier and Dilution Gases
Reactive precursors like silane are often diluted for safety and process control. They are mixed with an inert gas before entering the chamber.
- Common choices include nitrogen (N₂), argon (Ar), or helium (He).
- Diluting a gas like SiH₄ (e.g., 5% SiH₄ in 95% N₂) makes it more stable and allows for finer control over the deposition rate.
Plasma and Reactive Gases
Some gases are introduced to sustain the plasma or react with the primary precursor.
- Nitrogen (N₂) and ammonia (NH₃) act as both nitrogen precursors and reactive gases in the plasma.
- Oxygen (O₂) can be used as an oxygen source but is also a component of plasma cleaning gas mixtures.
Chamber Cleaning Gases
After deposition, a residue can build up on the chamber walls. A plasma cleaning step is used to remove it, ensuring process repeatability.
- A mixture of tetrafluoromethane (CF₄) and oxygen (O₂) is commonly used to etch away unwanted silicon-based deposits.
- Sulfur hexafluoride (SF₆) is another powerful etchant gas used for chamber cleaning.
Key Trade-offs in Gas Selection
Choosing the right gas mixture involves balancing deposition speed, film quality, and safety.
Film Quality vs. Deposition Rate
The choice of precursor can impact the final film. For example, using ammonia (NH₃) to deposit silicon nitride is fast but incorporates hydrogen into the film, which can affect its electrical properties. Using nitrogen (N₂) results in a denser, lower-hydrogen film but at a much slower deposition rate.
Safety and Handling
Many precursor gases are hazardous. Silane (SiH₄) is pyrophoric, meaning it can ignite spontaneously in air. This is why it is often purchased in diluted mixtures and handled with extreme care using specialized gas delivery systems.
Process Control and Repeatability
The purity of the source gases is paramount. Even trace contaminants can be incorporated into the film and degrade its performance. Likewise, the mass flow controllers that regulate gas flow must be highly precise to ensure the gas ratios are exactly what the recipe demands, run after run.
Making the Right Choice for Your Goal
Your choice of gas is dictated entirely by the material you need to create.
- If your primary focus is standard microelectronic insulation: You will use SiH₄ with either N₂O (for silicon dioxide) or NH₃ (for silicon nitride).
- If your primary focus is a hard, wear-resistant coating: You will use a hydrocarbon precursor like acetylene to deposit Diamond-Like Carbon (DLC).
- If your primary focus is process safety and fine control: You should specify diluted precursors (e.g., 5% SiH₄ in Ar) and ensure high-precision mass flow controllers are used.
- If your primary focus is equipment uptime and consistency: You must implement a robust chamber cleaning recipe using gases like CF₄/O₂ or SF₆ between deposition runs.
Ultimately, mastering the PECVD process is mastering the chemistry of its gases.
Summary Table:
| Film Type | Common Precursor Gases | Key Process Gases |
|---|---|---|
| Silicon Dioxide (SiO₂) | Silane (SiH₄) | Nitrous Oxide (N₂O), Argon (Ar) |
| Silicon Nitride (Si₃N₄) | Silane (SiH₄) | Ammonia (NH₃) or Nitrogen (N₂) |
| Diamond-Like Carbon (DLC) | Acetylene (C₂H₂), Methane (CH₄) | Argon (Ar), Hydrogen (H₂) |
| Chamber Cleaning | - | CF₄/O₂ mixture, Sulfur Hexafluoride (SF₆) |
Struggling to optimize your PECVD process for consistent, high-quality thin films?
At KINTEK, we understand that gas chemistry is the heart of PECVD. Our advanced Tube Furnaces and CVD/PECVD Systems are engineered for precise gas delivery and control, ensuring the repeatability your R&D or production demands. Leveraging our exceptional in-house manufacturing and deep customization capabilities, we can tailor a furnace solution to your exact gas recipes and safety requirements—whether you're depositing silicon nitride with ammonia or exploring novel materials.
Let's discuss how we can enhance your deposition process. Contact our experts today for a personalized consultation.
Visual Guide
Related Products
- Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
People Also Ask
- How does the microarc produced by ion discharge function? Enhance Coating Bonding Strength via Surface Activation
- What are some key features of the PECVD system? Unlock Low-Temp, High-Rate Thin Film Deposition
- What materials can be used as coatings in PECVD? Explore Versatile Thin-Film Solutions for Your Lab
- Why is gas flow rate important in PECVD? Master Film Growth and Quality Control
- How can PECVD process parameters be optimized? Master Film Quality and Deposition Efficiency
- Why is the vacuum reaction chamber critical for PECVD on titanium alloys? Precision Control for Superior Coatings
- How is PECVD applied in optical coatings? Enhance Light Control with Precision Films
- How does PECVD equipment work? Unlock Low-Temperature Thin Film Deposition



















