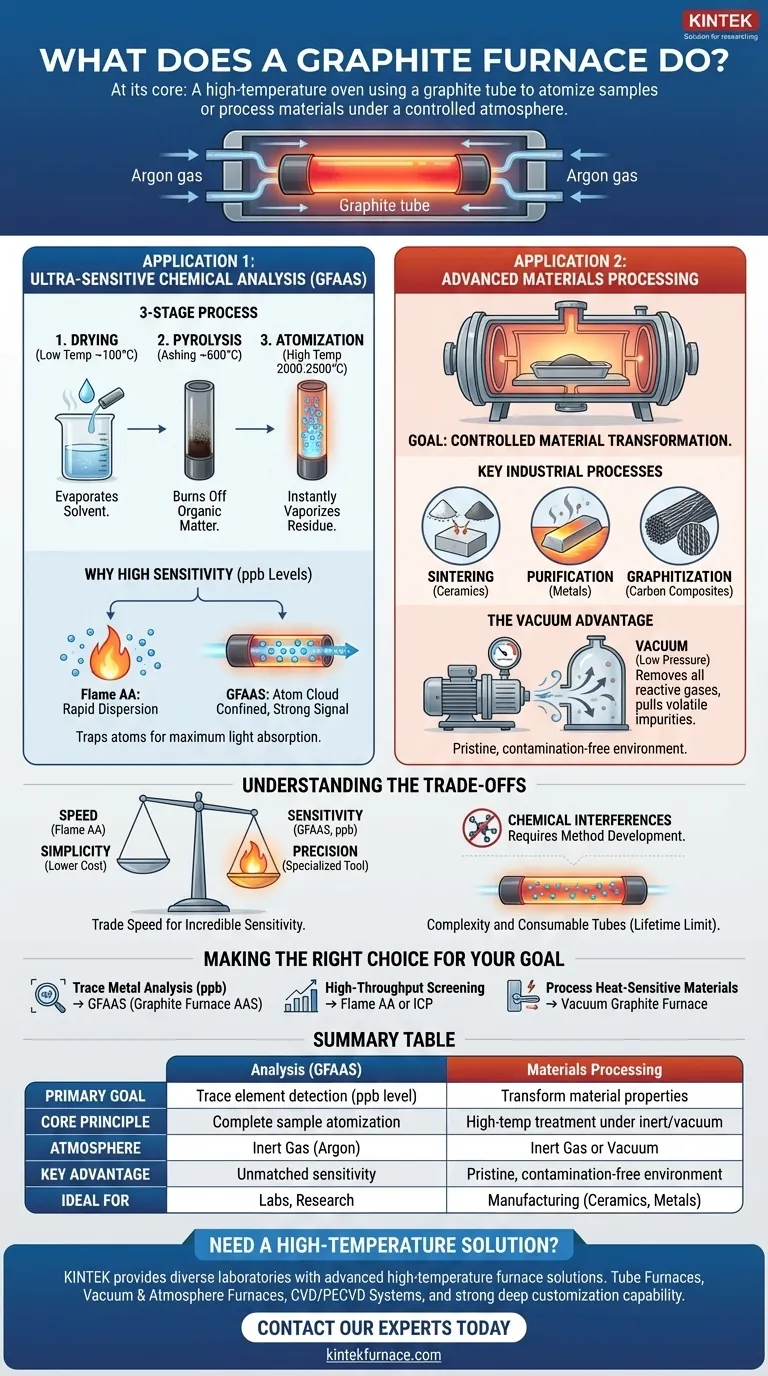
At its core, a graphite furnace is a specialized high-temperature oven that uses a graphite tube as its heating element. Its primary functions are to either prepare a sample for ultra-sensitive chemical analysis by vaporizing it into individual atoms or to process materials under a highly controlled, oxygen-free atmosphere.
The true value of a graphite furnace lies in its precision. By perfectly controlling temperature and atmosphere within a small, contained space, it allows for either the complete isolation of atoms for measurement or the high-purity processing of advanced materials.

The Core Function: High-Temperature Control
A graphite furnace's operation is based on a few key principles that enable its unique capabilities.
How It Heats
A small, hollow graphite tube is held between two electrodes. When a high current is passed through the electrodes, the electrical resistance of the graphite causes it to heat up rapidly, capable of reaching temperatures over 2500°C in seconds.
The Role of the Inert Atmosphere
The entire process occurs within a sealed chamber filled with an inert gas, almost always argon. This is critical because at such high temperatures, the graphite tube and the sample would instantly burn up (oxidize) if exposed to oxygen in the air. The argon gas protects the components and ensures the sample is heated, not incinerated.
Application 1: Ultra-Sensitive Chemical Analysis (GFAAS)
The most common use for a graphite furnace is in a technique called Graphite Furnace Atomic Absorption Spectroscopy (GFAAS), also known as Electrothermal Atomization (ETA).
The Goal: Complete Atomization
For chemical analysis, the furnace's job is to take a tiny liquid sample (a few microliters) and break it down completely. The goal is to strip away all solvents and break every chemical bond until all that remains is a tiny cloud of free, neutral atoms of the element you want to measure.
Why This Achieves High Sensitivity
Unlike a flame, which quickly disperses the sample, a graphite furnace traps this atom cloud inside the graphite tube for a second or two. A beam of light is passed through the tube, and because the atoms are so concentrated, they absorb a large amount of light, generating a strong, clear signal for the detector. This confinement is why GFAAS can detect elements at parts-per-billion (ppb) concentrations.
The Three-Stage Process
The furnace achieves atomization through a carefully programmed heating sequence:
- Drying: A low temperature (around 100°C) gently evaporates the solvent from the sample.
- Pyrolysis (Ashing): A higher intermediate temperature (several hundred degrees) burns off organic matter and other unwanted components of the sample matrix.
- Atomization: A rapid ramp to a very high temperature (2000-2500°C) instantly vaporizes the remaining residue and breaks the chemical bonds, creating the cloud of free atoms for measurement.
Application 2: Advanced Materials Processing
A different class of graphite furnaces, often larger and operating under a vacuum, is used in materials science and industry.
The Goal: Controlled Material Transformation
Here, the purpose isn't analysis but to fundamentally change a material's properties using heat. The furnace provides a pristine, high-temperature environment free from reactive gases that could contaminate the product.
Key Industrial Processes
These furnaces are essential for manufacturing advanced materials. Common applications include the sintering of ceramics, the purification of metals, or the graphitization of carbon composites, where precise heat treatment is necessary to achieve the desired material strength and purity.
The Advantage of a Vacuum
For materials processing, the furnace is often operated under a vacuum (low pressure) instead of just an argon atmosphere. Pulling a vacuum is the most effective way to remove all residual gases, especially oxygen, and can also help pull volatile impurities out of the material as it is being heated.
Understanding the Trade-offs
While powerful, a graphite furnace is a specialized tool with specific limitations.
Speed vs. Sensitivity
GFAAS is a sequential, one-sample-at-a-time technique. The heating cycle for a single sample can take a few minutes, making it much slower than other methods like flame AA. You trade speed for incredible sensitivity.
Complexity and Cost
Graphite furnaces are more complex instruments than their flame-based counterparts. Furthermore, the graphite tubes are consumable parts with a limited lifetime of a few hundred firings, adding to the operational cost.
Chemical Interferences
The sample's "matrix" (everything in the sample that isn't the element of interest) can sometimes interfere with the atomization process, leading to inaccurate results. Overcoming these "matrix effects" requires careful method development and expertise from the operator.
Making the Right Choice for Your Goal
Deciding to use a graphite furnace depends entirely on your objective.
- If your primary focus is trace metal analysis at parts-per-billion levels: A graphite furnace (GFAAS) is the definitive choice for achieving the required sensitivity and low detection limits.
- If your primary focus is high-throughput screening of many samples for major elements: A faster technique like Flame Atomic Absorption (FAA) or Inductively Coupled Plasma (ICP) is a more efficient solution.
- If your primary focus is processing heat-sensitive materials in an oxygen-free environment: A vacuum graphite furnace is the ideal industrial tool for ensuring product purity and desired material properties.
Ultimately, a graphite furnace is a tool of precision, enabling control at the atomic and material level that is otherwise unattainable.
Summary Table:
| Key Feature | Analysis (GFAAS) | Materials Processing |
|---|---|---|
| Primary Goal | Trace element detection (ppb level) | Transform material properties (sintering, purification, graphitization) |
| Core Principle | Complete sample atomization in a graphite tube | High-temperature treatment under inert gas or vacuum |
| Atmosphere | Inert Gas (Argon) | Inert Gas or Vacuum |
| Key Advantage | Unmatched sensitivity for tiny samples | Pristine, contamination-free environment |
| Ideal For | Labs requiring ultra-low detection limits | Manufacturing advanced materials like ceramics and metals |
Need a High-Temperature Solution Tailored to Your Specific Requirements?
Whether your lab demands the ultra-sensitive detection capabilities of a Graphite Furnace AAS system or the high-purity processing environment of a vacuum furnace for advanced materials, KINTEK has the expertise and manufacturing capability to deliver.
Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with advanced high-temperature furnace solutions. Our product line, including Tube Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, is complemented by our strong deep customization capability to precisely meet your unique experimental or production requirements.
Contact our experts today to discuss how we can help you achieve precise thermal processing and analysis results.
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is an example of a material prepared using a tube furnace? Master Precise Material Synthesis
- How does a vertical tube furnace achieve precise temperature control? Unlock Superior Thermal Stability for Your Lab
- How is a Vertical Tube Furnace used for fuel dust ignition studies? Model Industrial Combustion with Precision
- What safety measures are essential when operating a lab tube furnace? A Guide to Preventing Accidents
- How is a high-temperature tube furnace utilized in the synthesis of MoO2/MWCNTs nanocomposites? Precision Guide



















