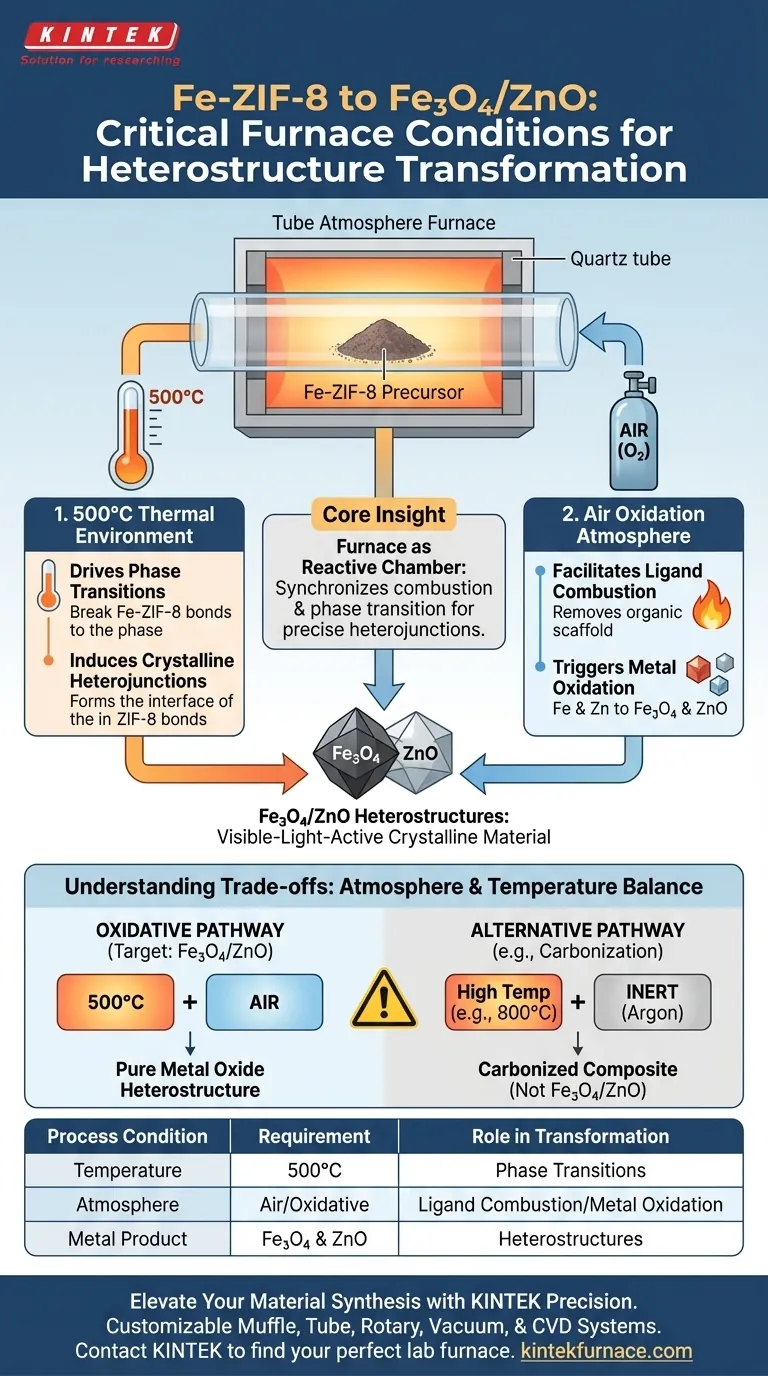
To transform Fe-ZIF-8 into Fe3O4/ZnO heterostructures, a tube atmosphere furnace must provide two critical conditions: a consistent 500°C thermal environment and a strictly controlled air oxidation atmosphere. This specific combination facilitates the simultaneous removal of organic components and the oxidation of metal centers required to form the final heterostructure.
Core Insight: The tube furnace functions not just as a heater, but as a reactive chamber that synchronizes the combustion of organic ligands with the phase transition of metal ions, resulting in precise, visible-light-active crystalline heterojunctions.

The Role of the Oxidative Environment
While many furnace applications require inert atmospheres (such as Argon) to prevent oxidation, this specific transformation relies on the presence of oxygen.
Facilitating Ligand Combustion
The primary function of the air atmosphere is to drive the complete thermal decomposition of the organic ligands found within the ZIF-8 framework.
In an inert environment, these ligands might carbonize. However, the airflow in the tube furnace ensures they undergo combustion, effectively removing the organic "scaffold" to leave behind the metallic components.
Triggering Metal Oxidation
Simultaneously, the oxygen in the air reacts with the iron (Fe) and zinc (Zn) ions released from the decomposing framework.
This transforms the metal ions into their oxide forms—specifically Fe3O4 (magnetite) and ZnO (zinc oxide). This chemical change is impossible without a consistent supply of oxygen during heating.
Thermal Precision at 500°C
Temperature control is the second pillar of this process. The furnace must maintain a steady 500°C to balance destruction and creation.
Driving Phase Transitions
At this specific temperature, the energy is sufficient to break the chemical bonds of the ZIF-8 precursor.
It causes the organic components to burn off while providing the activation energy needed for the metal ions to undergo a phase transition into stable crystalline oxides.
Inducing Crystalline Heterojunctions
The thermal environment does more than just oxidize; it structures the material.
The 500°C heat induces the formation of a crystalline heterojunction between the Fe3O4 and ZnO. This precise interface is what grants the final material its visible light activity and photocatalytic properties.
Understanding the Trade-offs
Using a tube atmosphere furnace requires understanding the delicate balance between atmosphere and temperature.
The Risk of Incorrect Atmosphere
It is critical not to confuse this process with carbonization protocols.
For example, when processing materials like t-BTO@C, an inert argon atmosphere is used to create conductive carbon layers. If you were to apply that inert atmosphere to Fe-ZIF-8, you would likely produce a carbonized composite rather than the desired pure metal oxide heterostructure.
Temperature Sensitivity
DEviating significantly from 500°C alters the material properties.
Temperatures that are too low may result in incomplete ligand decomposition, leaving impurities. Temperatures that are excessive could lead to unrestricted grain growth, damaging the delicate heterojunctions required for performance.
Making the Right Choice for Your Goal
When configuring your tube furnace, your settings dictate the chemical pathway.
- If your primary focus is synthesizing Fe3O4/ZnO Heterostructures: Ensure the furnace is set to 500°C with a continuous air atmosphere to drive oxidation and ligand combustion.
- If your primary focus is Carbonization (e.g., t-BTO@C): You must switch to an inert atmosphere (Argon) and higher temperatures (e.g., 800°C) to prevent oxidation and preserve conductive carbon layers.
Success depends on aligning the furnace atmosphere—oxidative or inert—strictly with the chemical transformation you intend to induce.
Summary Table:
| Process Condition | Requirement | Role in Fe-ZIF-8 Transformation |
|---|---|---|
| Temperature | 500°C | Drives phase transitions and induces crystalline heterojunctions |
| Atmosphere | Air (Oxidative) | Facilitates ligand combustion and triggers metal center oxidation |
| Metal Product | Fe3O4 & ZnO | Forms stable metal oxide heterostructures for photocatalysis |
| Alternative | Argon (Inert) | Used for carbonization (e.g., t-BTO@C) instead of oxidation |
Elevate Your Material Synthesis with KINTEK Precision
Are you looking to achieve precise crystalline heterojunctions or specialized carbonization layers? KINTEK provides industry-leading thermal solutions designed for the most demanding research applications. Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your specific temperature and atmospheric requirements.
Don't let inconsistent thermal environments compromise your material science results. Partner with KINTEK to ensure absolute control over your chemical transformations.
Contact Our Technical Experts Today to find the perfect lab high-temp furnace for your unique needs!
Visual Guide
References
- Sumiyyah Sabar, Hiromi Yamashita. Construction of Fe <sub>3</sub> O <sub>4</sub> /ZnO heterostructure photocatalysts derived from Fe-doped ZIF-8 for enhanced photocatalytic degradation of tetracycline and hydrogen peroxide production. DOI: 10.1039/d5nj00407a
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What are the applications of inert atmosphere furnaces? Essential for Metal Processing, Electronics, and Additive Manufacturing
- What are the key components of an inert atmosphere furnace? Essential Parts for Contamination-Free Heating
- Why is an industrial calcination furnace required to process carbon-supported nickel catalysts at 600°C in nitrogen?
- What are the four main types of controlled atmospheres used in these furnaces? Optimize Your Heat Treatment Processes
- What are the common gases and vapors used in furnace atmospheres and their roles? Optimize Your Heat Treatment Process
- Why is the temperature control of a high-precision resistance furnace essential for B4C/Al composites? Gain Control
- What role does a vacuum or atmosphere tube furnace play in the sintering process of Al6061/B4C composites?
- What are the operational considerations for atmosphere furnaces? Master Precise Control for Safe, Efficient Results



















