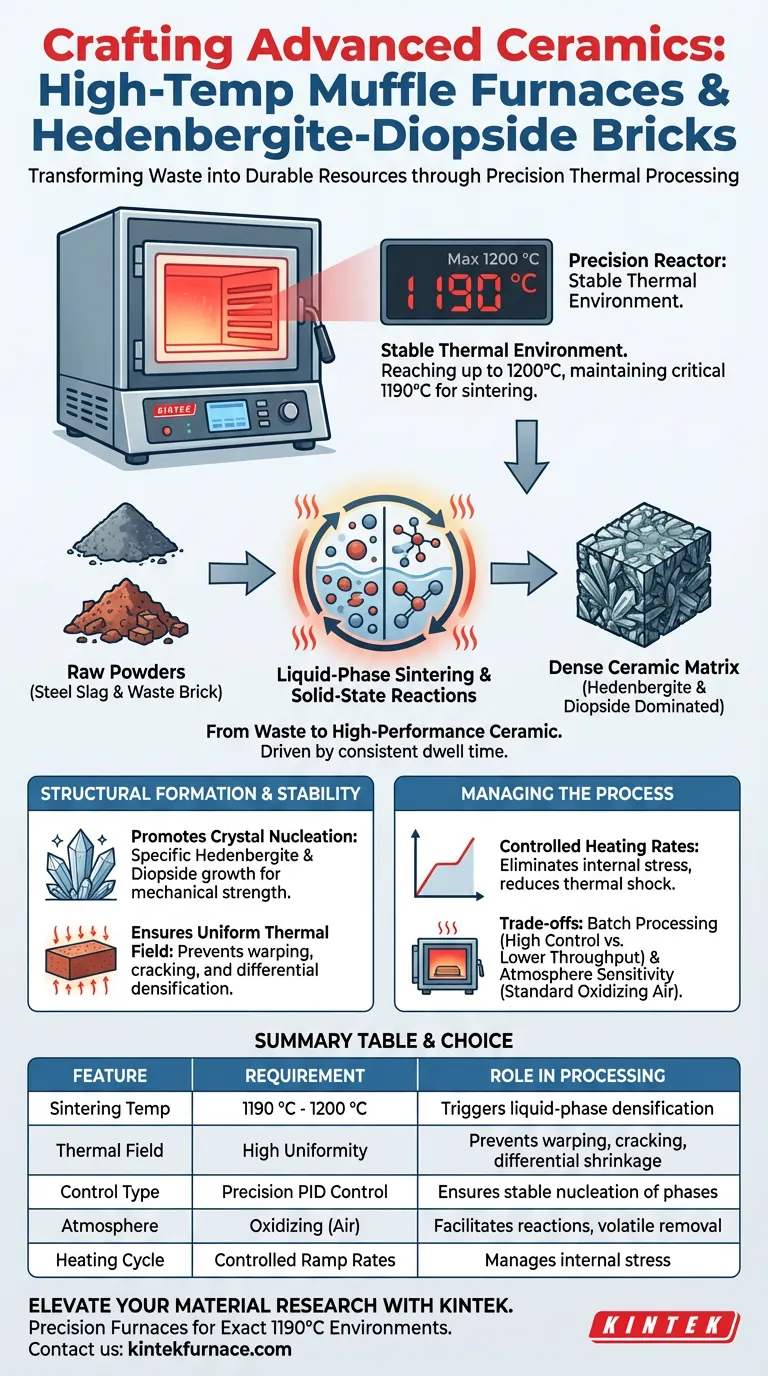
A high-temperature muffle furnace acts as a precision reactor for ceramic synthesis. It provides a stable thermal environment capable of reaching 1200 °C, specifically maintaining a critical sintering temperature of 1190 °C. This exact thermal regime is required to drive the solid-state reactions and liquid-phase sintering that transform raw powders—specifically steel slag and waste brick—into a dense ceramic matrix dominated by hedenbergite and diopside phases.
The muffle furnace’s primary role is to sustain a uniform thermal field at 1190 °C, which triggers the liquid-phase sintering necessary to bond waste materials. This controlled environment promotes the specific nucleation and growth of hedenbergite and diopside crystals, ensuring the final product achieves high structural density and durability.

The Thermal Environment for Phase Transformation
Achieving the Critical Sintering Point
To synthesize ceramics with these specific mineral phases, the furnace must provide rigorous temperature control up to 1200 °C.
The primary reference indicates that the optimal sintering temperature for this material system is 1190 °C. At this precise threshold, the thermal energy is sufficient to activate the raw materials without causing structural collapse or melting.
Facilitating Liquid-Phase Sintering
The muffle furnace creates the thermodynamic conditions necessary for liquid-phase sintering.
This process occurs when the powder mixture—comprising steel slag and waste brick—forms a liquid phase at high temperatures. This liquid promotes particle rearrangement and densification, acting as the medium through which the ceramic structure consolidates.
Driving Solid-State Reactions
Beyond simple heating, the furnace environment facilitates complex solid-state reactions between the different powder components.
These reactions are chemically intricate and rely on the furnace's ability to maintain a consistent temperature over a set duration (dwell time). This stability ensures that the reactants have sufficient time to diffuse and form new chemical bonds.
Structural Formation and Stability
Promoting Crystal Nucleation
The defining characteristic of these bricks is the presence of hedenbergite and diopside as the main crystalline phases.
The thermal stability provided by the muffle furnace promotes the nucleation and subsequent growth of these crystals, along with magnetite. The controlled heat ensures these phases develop fully, which is directly correlated to the mechanical strength of the brick.
Ensuring Uniform Thermal Fields
While the primary focus is the peak temperature, the furnace also provides a uniform thermal field (a concept supported by supplementary references on ceramic sintering).
A uniform field ensures that the entire brick is exposed to the same temperature simultaneously. This synchronization prevents differential densification, which leads to warping or cracking.
Managing Internal Stress
The furnace allows for controlled heating rates, which helps in the elimination of internal stresses.
By raising the temperature gradually and maintaining uniformity, the furnace prevents thermal shock. This helps reduce volume shrinkage rates and mitigates the risk of defects forming during the cooling phase.
Understanding the Trade-offs
Batch Processing Limitations
Muffle furnaces are typically batch-processing units. While they offer superior control for research and high-value synthesis, they may have lower throughput compared to continuous tunnel kilns used in mass production.
Sensitivity to Atmosphere
While muffle furnaces generally provide a stable oxidizing atmosphere (air), they rely on the ambient environment unless specifically equipped with gas controls.
For hedenbergite and diopside formation, a standard oxidizing environment is generally beneficial. However, if the raw material contains high organic content or carbonates, the furnace must be managed carefully to allow for the complete escape of volatiles before the pores close during sintering.
Making the Right Choice for Your Goal
To successfully prepare ceramic bricks with hedenbergite and diopside phases, you must align the furnace capabilities with your specific processing objectives.
- If your primary focus is Phase Purity: Prioritize a furnace with high-precision temperature control to hold exactly at 1190 °C, ensuring the complete crystallization of hedenbergite and diopside without overheating.
- If your primary focus is Structural Density: Focus on the furnace's ability to maintain a uniform thermal field to facilitate even liquid-phase sintering and minimize porosity across the entire brick volume.
- If your primary focus is Material Recycling: Ensure the furnace has adequate ventilation or exhaust systems to handle the off-gassing of volatiles from the steel slag and waste brick powders during the pre-sintering ramp-up.
Success in this synthesis relies not just on reaching high heat, but on the stability and uniformity of the thermal environment at the critical 1190 °C threshold.
Summary Table:
| Feature | Requirement for Synthesis | Role in Ceramic Processing |
|---|---|---|
| Sintering Temp | 1190 °C - 1200 °C | Triggers liquid-phase sintering for densification. |
| Thermal Field | High Uniformity | Prevents warping, cracking, and differential shrinkage. |
| Control Type | Precision PID Control | Ensures stable nucleation of hedenbergite & diopside. |
| Atmosphere | Oxidizing (Air) | Facilitates solid-state reactions and volatile removal. |
| Heating Cycle | Controlled Ramp Rates | Manages internal stress and reduces thermal shock risk. |
Elevate Your Material Research with KINTEK
Precision is the difference between a failed sample and a high-performance ceramic. At KINTEK, we understand that synthesizing phases like hedenbergite requires absolute thermal stability. Our laboratory high-temperature furnaces—including Muffle, Tube, Rotary, Vacuum, and CVD systems—are engineered to deliver the exact 1190 °C environment your research demands.
Why choose KINTEK?
- Expert R&D: Optimized thermal fields for uniform crystal growth.
- Customizable Solutions: Tailored furnace dimensions and gas controls to meet unique synthesis needs.
- Industrial Durability: Built to handle waste-to-resource materials like steel slag and waste brick.
Contact us today to find the perfect furnace for your lab!
Visual Guide
References
- Ying Ji, Qianqian Sha. Preparation and Performance of Ceramic Tiles with Steel Slag and Waste Clay Bricks. DOI: 10.3390/ma17081755
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How to keep samples in muffle furnace? A Step-by-Step Guide for Safe & Accurate Results
- What role does a laboratory high-temperature furnace play during the pyrolysis stage of UHTCMCs?
- What are the steps to operate a box muffle furnace? Master Safe and Efficient Heating Processes
- How do muffle furnaces help pharmaceutical companies comply with regulatory standards? Ensure Precise QC for FDA/EMA Approval
- What are the common applications of benchtop furnaces? Unlock Precision in Materials Science and More
- How do laboratory high-temperature furnaces assist in determining the annealing temperatures? Replicate Ancient Metalwork
- How does a laboratory muffle furnace facilitate the biomass carbonization process? Achieve Precise Biochar Production
- What is sintering, and how is a muffle furnace used in this process? Unlock Precision in Material Bonding



















