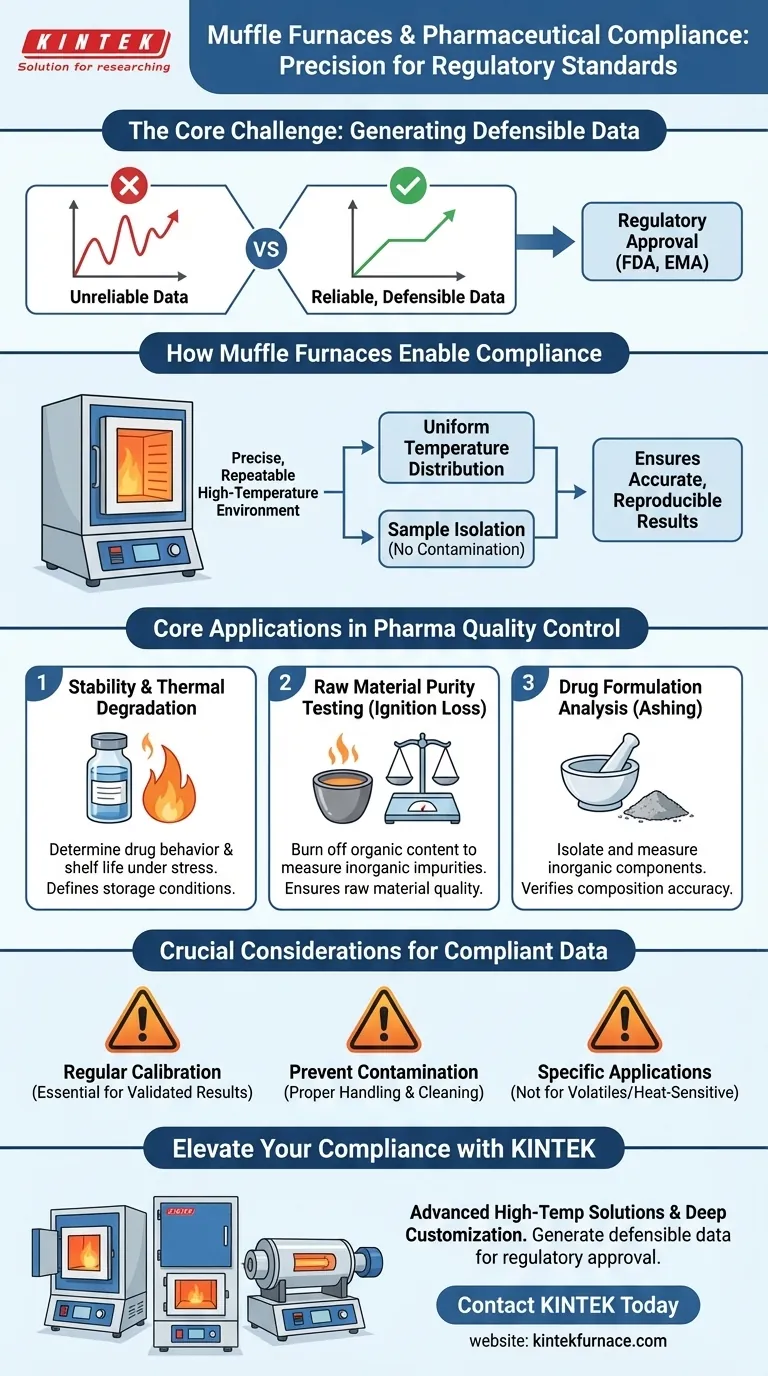
In short, muffle furnaces enable pharmaceutical companies to comply with regulatory standards by providing a precise and repeatable high-temperature environment. This controlled environment is essential for conducting specific quality control tests on raw materials and finished drugs, generating the reliable data required by bodies like the FDA and EMA to prove a product's safety, purity, and stability.
The core challenge in pharmaceutical compliance is not just performing tests, but proving those tests are accurate and reproducible. Muffle furnaces are a critical tool for this, creating the stable, high-temperature conditions necessary to generate defensible data for regulatory submission and quality assurance.

The Foundation of Compliance: Repeatable Analysis
A muffle furnace is fundamentally a high-temperature oven, but its design is what makes it critical for regulated industries.
What is a Muffle Furnace?
A muffle furnace uses an insulated outer chamber to heat an inner chamber, known as the muffle. This design isolates the sample from direct contact with the heating elements.
This separation prevents contamination and ensures exceptionally uniform temperature distribution, which is the cornerstone of reliable testing.
Why Precision Temperature is Non-Negotiable
Regulatory bodies like the FDA (Food and Drug Administration) and EMA (European Medicines Agency) demand data that is both accurate and reproducible.
If a quality test yields different results each time it's run, the data is useless for proving a drug's quality. Muffle furnaces provide the thermal stability needed to ensure test results are consistent and trustworthy.
Core Applications in Pharmaceutical Quality Control
Muffle furnaces are not used for all tests, but they are indispensable for specific high-temperature analyses that form a key part of a regulatory submission.
Stability and Thermal Degradation Studies
Regulators need to know how a drug behaves under stress, including high heat. A muffle furnace is used to subject a drug to controlled, elevated temperatures.
By analyzing the sample afterward, scientists can determine its degradation point and overall thermal stability, which is vital information for defining storage conditions and shelf life.
Raw Material Purity Testing
The quality of a final drug product depends entirely on the quality of its raw materials. Muffle furnaces are used in a process called ignition loss testing.
In this test, a sample is weighed, heated to a very high temperature to burn off all organic content, and then weighed again. The difference in weight reveals the amount of inorganic impurities, ensuring the raw material meets stringent purity specifications before it ever enters the manufacturing line.
Analysis of Drug Formulations
These furnaces also help in analyzing the composition of final drug formulations. Certain analytical procedures require a sample to be reduced to ash (ashing) to isolate and measure its inorganic components.
This process confirms that the correct minerals or inorganic compounds are present in the specified quantities, verifying the accuracy of the formulation.
Understanding the Trade-offs and Limitations
While essential, a muffle furnace is a tool that must be used correctly to provide compliant data. Misuse can invalidate results and jeopardize regulatory approval.
The Critical Role of Calibration
An uncalibrated furnace is a liability. If the temperature display reads 500°C but the actual temperature is 520°C, every test performed is based on false parameters.
Regular, documented calibration is a non-negotiable regulatory requirement. Without it, all data generated from the furnace can be challenged and rejected by auditors.
Potential for Sample Contamination
While the muffle design minimizes contamination from heating elements, poor laboratory practices can still compromise a sample.
Using unclean crucibles or failing to follow proper handling procedures can introduce foreign materials, skewing the results of sensitive purity tests.
Not a Universal Solution
A muffle furnace is a specialized instrument for high-temperature applications, primarily involving inorganic analysis and thermal degradation.
It is not suitable for analyzing volatile compounds or substances that are destroyed by heat, which require different analytical techniques like chromatography or spectroscopy.
How to Apply This to Your Project
Your goal determines how you leverage a muffle furnace for compliance. The key is to connect the specific function of the furnace to a specific regulatory requirement.
- If your primary focus is Quality Control: Use the furnace for routine batch release testing, specifically ignition loss tests on raw materials and ashing procedures on finished products to verify composition.
- If your primary focus is Research & Development: Use the furnace to conduct thermal degradation studies and material characterization to establish the foundational stability data for a new drug application.
- If your primary focus is Manufacturing Process Development: Use the furnace for high-temperature processes like sintering to create consistent drug delivery systems or medical implants, ensuring the final product meets design specifications.
Ultimately, mastering the use of a muffle furnace is a key step in transforming laboratory work into proof of quality that regulators can trust.
Summary Table:
| Application | Regulatory Benefit | Key Function |
|---|---|---|
| Stability & Thermal Degradation Studies | Defines drug shelf life and storage conditions | Controlled heating to analyze degradation |
| Raw Material Purity Testing | Ensures raw material quality meets standards | Ignition loss testing to measure impurities |
| Drug Formulation Analysis | Verifies composition accuracy in final products | Ashing to isolate and quantify inorganic components |
Elevate your pharmaceutical compliance with KINTEK's advanced high-temperature furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with reliable tools like Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures precise alignment with your unique experimental needs, helping you generate defensible data for regulatory approvals. Contact us today to discuss how our furnaces can enhance your quality control and streamline compliance processes!
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- Why is re-calcination in a muffle furnace necessary for photocatalysts? Restore Efficiency via Thermal Oxidation
- What key step does a Muffle Furnace perform in the determination of mineral content in Tenebrio molitor larvae?
- What is the function of a muffle furnace in SiCf/Al-Mg pretreatment? Optimize Fiber Bonding with Thermal De-sizing
- What is the core function of a muffle furnace in CuO nanoparticle synthesis? Achieve Precision Calcination
- What is the primary function of a high-temperature box resistance furnace? Optimize Superalloy Homogenization



















