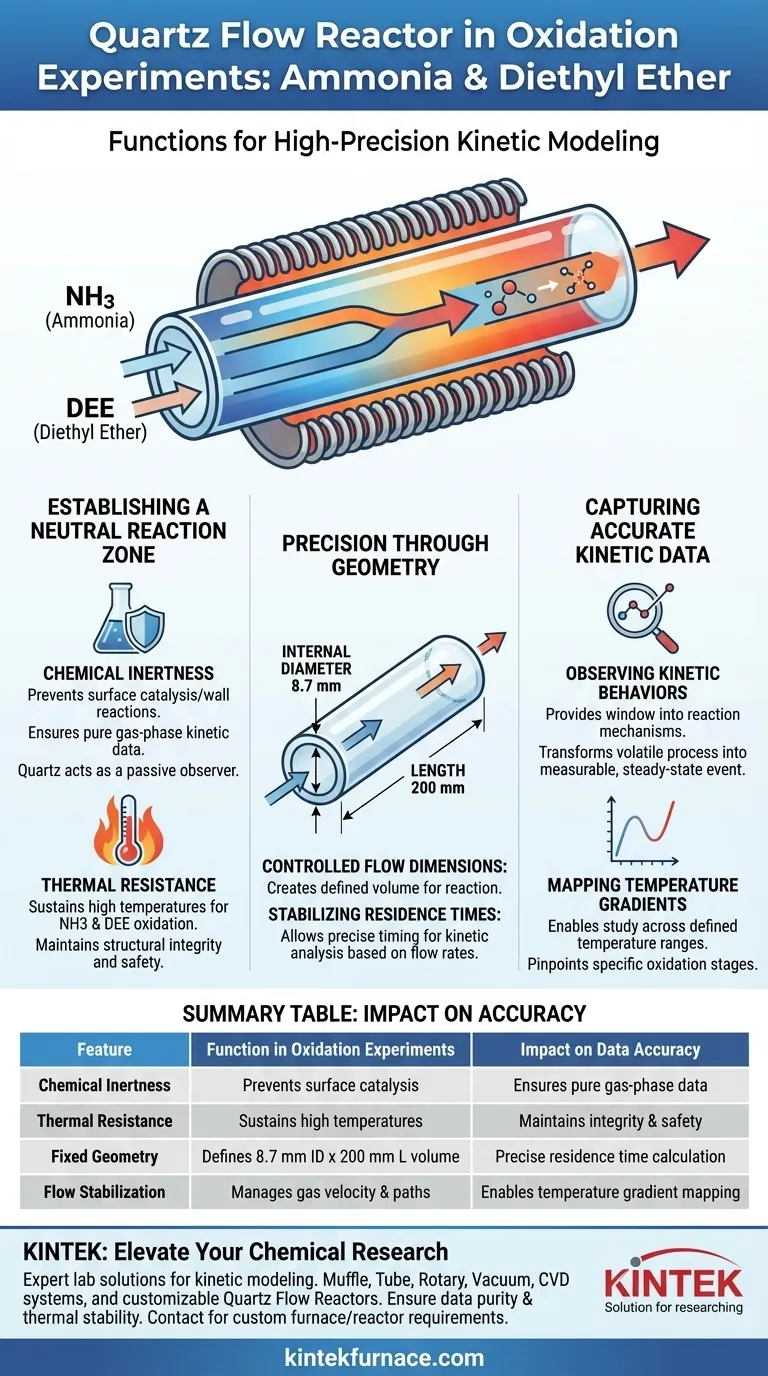
The Quartz Flow Reactor acts as the foundational vessel for conducting high-precision oxidation experiments involving ammonia (NH3) and diethyl ether (DEE). By leveraging the material's inherent chemical inertness and thermal resistance, this device creates a controlled flow environment essential for isolating specific reaction variables.
The reactor’s primary function is to stabilize gas residence times within a chemically neutral environment, allowing researchers to observe accurate kinetic behaviors across specific temperature gradients without interference from the vessel itself.

Establishing a Neutral Reaction Zone
Ensuring Chemical Inertness
In oxidation studies involving reactive compounds like ammonia and diethyl ether, the reaction vessel must remain a passive observer. Quartz is utilized specifically for its chemical inertness, ensuring that the material does not act as a catalyst or reactant. This guarantees that the experimental data reflects only the interaction between the gases, rather than surface reactions with the reactor walls.
Withstanding High Thermal Loads
Oxidation experiments frequently demand elevated temperatures to initiate and sustain chemical changes. The reactor utilizes high-temperature resistance to maintain structural integrity under these harsh conditions. This stability is vital for ensuring safety and consistency throughout the heating process.
Precision Through Reactor Geometry
Controlled Flow Dimensions
The physical design of the reactor is not arbitrary; it is engineered to manage gas flow characteristics. Specific dimensions, such as an 8.7 mm internal diameter and a 200 mm length, are employed to create a defined volume for the reaction. This geometry ensures the gases follow a predictable path through the heated zone.
Stabilizing Residence Times
For accurate kinetic analysis, researchers must control exactly how long the reactants are exposed to heat. The reactor's specific dimensions allow for the maintenance of stable gas residence times at designated flow rates. This precise timing is the variable that allows researchers to calculate reaction speeds effectively.
Capturing Accurate Kinetic Data
Observing Kinetic Behaviors
The ultimate purpose of the reactor is to provide a window into the reaction mechanisms of NH3 and DEE. By stabilizing the environment, the reactor allows for the accurate observation of reaction kinetic behaviors. It transforms a volatile chemical process into a measurable, steady-state event.
Mapping Temperature Gradients
Chemical behaviors change distinctively as temperatures rise or fall. The Quartz Flow Reactor enables the study of these reactions across defined temperature gradients. This capability allows scientists to pinpoint exactly at what temperatures specific oxidation stages occur.
Understanding Operational Constraints
The Importance of Dimensional Accuracy
While the reactor enables precision, it relies heavily on the exactness of its design. The ability to calculate residence time is directly tied to the fixed volume provided by the 8.7 mm diameter and 200 mm length. Any deviation in these dimensions or instability in the flow rate will compromise the accuracy of the kinetic data collected.
Making the Right Choice for Your Experiment
To maximize the utility of a Quartz Flow Reactor in oxidation studies, align your experimental setup with the reactor's physical properties:
- If your primary focus is data purity: Rely on the quartz construction to eliminate surface catalysis and ensure that all observed oxidation is gas-phase only.
- If your primary focus is kinetic modeling: Strictly calibrate your gas flow rates against the reactor's internal dimensions (8.7 mm x 200 mm) to achieve the exact residence times required for your calculations.
By controlling the thermal and physical environment, this reactor turns complex chemical chaos into quantifiable scientific insight.
Summary Table:
| Feature | Function in Oxidation Experiments | Impact on Data Accuracy |
|---|---|---|
| Chemical Inertness | Prevents surface catalysis/wall reactions | Ensures pure gas-phase kinetic data |
| Thermal Resistance | Sustains high temperatures for NH3/DEE | Maintains structural integrity & safety |
| Fixed Geometry | Defines 8.7 mm ID x 200 mm L volume | Allows precise residence time calculation |
| Flow Stabilization | Manages gas velocity and paths | Enables mapping of temperature gradients |
Elevate Your Chemical Research with KINTEK
Ready to achieve unmatched precision in your oxidation studies? KINTEK provides high-performance lab solutions tailored for complex kinetic modeling. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized Quartz Flow Reactors customizable for your unique experimental needs.
Ensure data purity and thermal stability today. Contact our specialists now to discuss your custom furnace or reactor requirements!
Visual Guide
References
- Adrián Ruiz-Gutiérrez, María U. Alzueta. A flow reactor study of NH<sub>3</sub>/DEE oxidation. DOI: 10.26754/jji-i3a.202511914
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What function do graphite chill plates or chill rings perform? Master Single-Crystal Blade Directional Solidification
- What is the function of high-purity alumina crucibles in NRBBO:Eu2+ sintering? Ensure Pure Phosphor Synthesis
- What are the advantages of using a two-color pyrometer? Precision Sensing for Ultra-High-Temperature Furnaces
- Why is a quartz tube utilized as the primary reaction vessel? Optimize Microwave-Assisted Metal Recovery Efficiency
- Why are evaporators and condensers required for zirconium tetrachloride purification? Mastering Nuclear-Grade Standards
- Why Is a High-Precision Heating and Stirring Platform Necessary for ZnO Sol-Gel Synthesis? Achieve Perfect Nanoparticles
- Why are high-purity alumina boats utilized as precursor containers in MoS2 synthesis? Ensure High-Quality 2D Materials
- What are the advantages of using graphite for Sb2S3 sulfurization? Enhancing Thermal Precision and Safety



















